arsenic removal
Reading time:general
Up until the year 2000, the European standard for drinking water was 50 µg · L–1 of As, a target that was within the reach of clarification or carbonate removal treatment in most cases. Since this standard was modified (1998 European directive 98/83/CE), the limit has been lowered to 10 µg · L–1 of As, and this has led to the development of more specific processes, especially in the case of water that had previously complied and that did not call for any other treatment.
Depending on the case, one of the following processes can be utilised which, unless otherwise specified, unless otherwise specified, require As(III) to be oxidised to produce As(V) using an oxidant (such as chlorine):
- coagulation – flocculation, at a pH ≤ 7 if possible; all studies have shown that ferric salts performed better than aluminium salts. Depending on the initial As level and on operating conditions, demand can fluctuate between 10 and 100 g FeCℓ3 per m³ (testing required in each case); depending on the amount to be used, the system will include either clarification or direct filtration through a sand or dual media filter after a contact time of a few minutes;
- adsorption over activated alumina, regenerated using sodium hydroxide and hydrochloric acid (preferably sulphuric acid due to the competition with sulphate ions); possible but not cost effective;
- carbonate removal using lime with magnesium oxide precipitation, requiring a pH of approximately 11, preventing this treatment from becoming easily widespread;
- biological iron removal, when the water to be treated is groundwater and contains dissolved iron; bacterial oxidation also acts on the As(III), which therefore renders preliminary chemical oxidation superfluous in this case; on the other hand, the raw water has to be enriched with divalent iron (by injecting FeSO4) in the event of an inadequate Fe/As ratio.
Of the other potential processes, some were not sufficiently effective (e.g. PAC, GAC ) or not specific (e.g. RO or NF membranes), while others have not been sufficiently tested (e.g. filtration through iron or manganese oxides), pending the development of an oxidised iron based granular product.
the GEH process
The filtering medium is an iron oxihydroxide that has the property of fixing large quantities of As(V) without noticeably changing the water’s overall salinity (TAC, Cℓ–, SO42–…). In France, SUEZ has obtained product approval as well as the authorisation to use this process for drinking water.
Depending on the properties of the water and on its initial arsenic concentration, there is a range of potential systems as shown in figure 40.

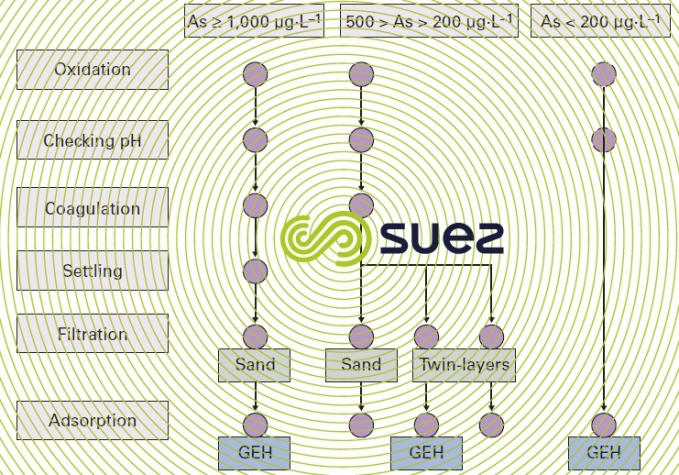

The pH is exceedingly important and it is best to remain within 6.5 to 7.5 in order to maximise capacity between two reloads.
Under present conditions, GEH cannot be regenerated but merely replaced every 1 to 3 years.
Therefore, the advantage of this process comes from the simplicity of its utilisation because pre-oxidation sometimes proves to be ineffective and, in the case of direct adsorption, all that is required is decompaction using water every fifteen days and monitoring the arsenic levels in the treated water; furthermore, as indicated by figure 41, the leak is very gradual, making this an extremely reliable process.

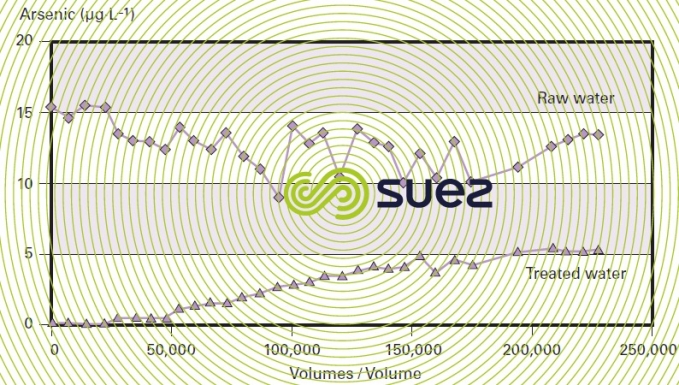

In all cases, it is advisable to carry out pilot trials in order to determine optimum utilisation conditions.
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












