general principles
Reading time:Solid reagents used in water treatment applications are usually made into a solution or a suspension in water, facilitating:
- transfer to the point where the product is to be used;
- mixing with the fluid to be processed;
- and, quite often, dispensing regularity and precision.
The preparation of routine reagent suspensions and solutions (sodium chloride, aluminium sulphate, ferric chloride, slaked lime) does not present any particular problems when the reagents have a regular particle size and a controlled level of impurities. Should this not be the case, special provisions must be taken to:
- eliminate impurities;
- use reagents in blocks or solidified reagents.
In general, provisions for preparing solutions or suspensions must be appropriate to the actual quality of the solid reagents available locally (particle size and impurities).
Furthermore, special precautions are required when maintaining certain fouling and/or scaling reagents in suspension, such as lime or activated carbon.
The energy required for producing a solution or suspension from solid reagents is mainly supplied by:
- mechanical agitation (figure 5);

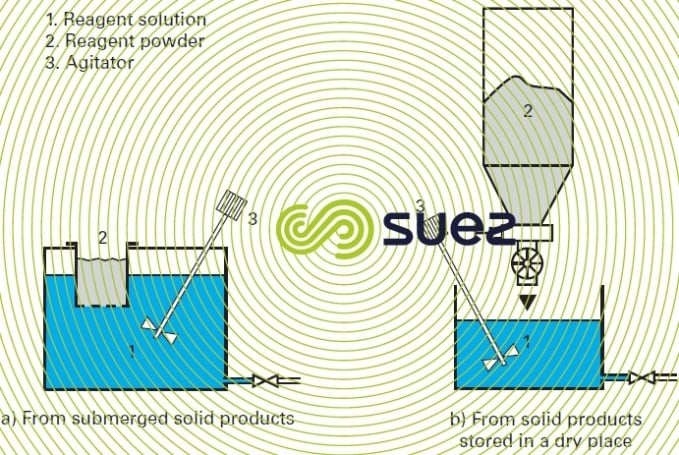

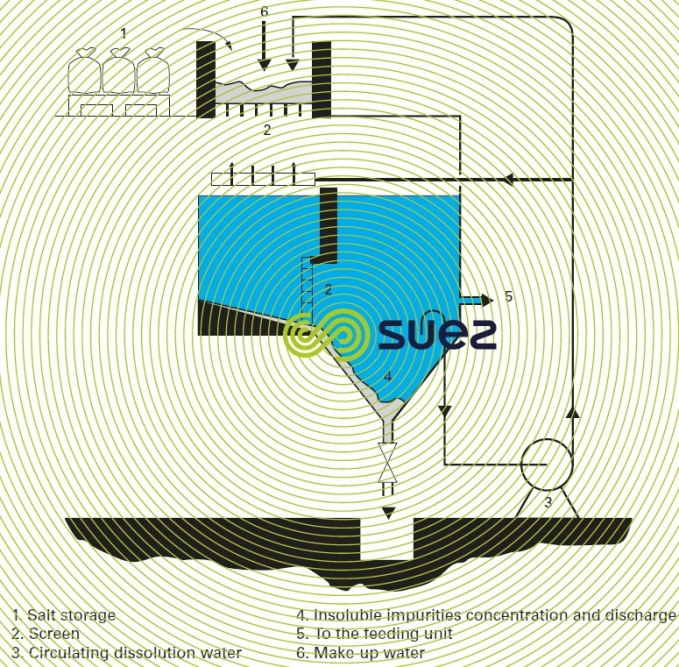
- forced recirculation of the dissolution water (figure 6).
This energy must be supplied on a continuous basis in the case of suspensions; in the case of solutions, it is normally only used while the solution is being prepared.
Preparing a tank of reagent must not affect treatment (alternating or separate tanks for preparation and feeding).


Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later













