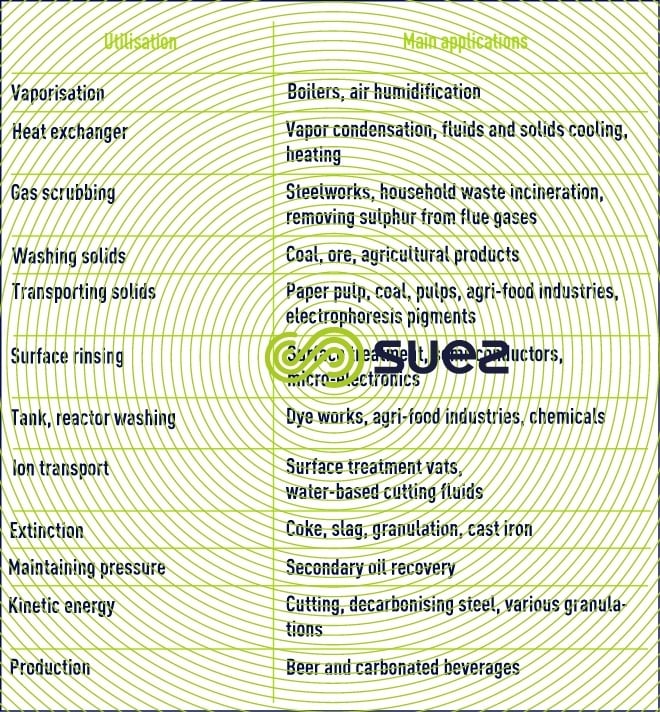
water uses and target qualities
Reading time:The industry extends to a widely variable range of uses in terms of quantity and quality.
Additionally, the combination of increasingly stringent environment and resource protection provisions (discharge standards, taxes …) require industry to manage its water ever more meticulously, more and more frequently with the use of cascade and/or recycling systems, whether within the workshop or throughout an industrial site. This explains the very wide variations in water sampling found from one site to another.
elementary water utilisation
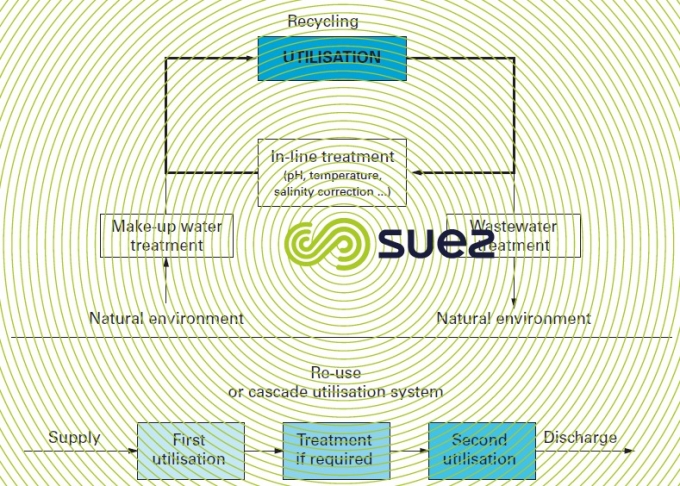
Water can be intended (figure 11):
- for one single utilisation:
- in an once-through circuit or direct make-up;
- or through recycling with or without deterioration;
- two different and successive utilisations or re-use or even utilisation in cascades.







recycling without water deterioration
This recycling consists in using the same water several times for the same utilisation that creates very little pollution. Make-up water compensates for various liquid losses (leaks, water that is drawn off, sludge water…) or vapour losses when, as frequently occurs, there is evaporation. Normally, water does not suffer noticeable deterioration through an influx of outside ions, gas solubilisation or through the dissolving of mineral or organic matter as the water is used. Only the original salts will become concentrated as the result of evaporation.
The two current examples of this clean recycling are cooling using an atmospheric coolant or "open recirculating circuit" (see cooling circuits), and boiler water where the condensates are returned to the inlet.
Recycling rate R: ratio of the circulation flow rate Q over the make-up water flow A can be high and, consequently, the increased concentration of the various salts as the result of evaporation can be high; this requires the least soluble salts to be removed in advance from the make-up water and the water circuit to undergo a deconcentration blowdown.
Estimating and maintaining concentration level C is crucial and must be undertaken by adjusting the deconcentration or blowdown flowrate D.
As indicated by figure 12, C can be calculated from the salt balance by measuring S and s or by calculating C from the flow rate:
- the water balance provides A = E + D;
- the salt balance provides As = DE.

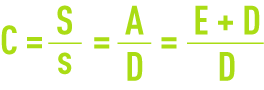
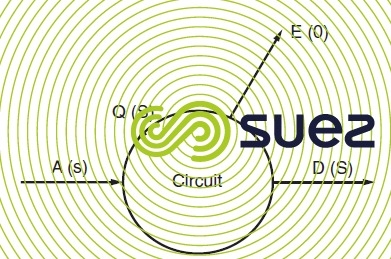
A = make-up water flow;
D = concentration flow (deliberate or in the form of leaks);
Q = flow through circuit;
E = evaporation flow;
S = circuit salinity and s is make-up water salinity.


In a open recirculating cooling circuit, C can vary from 1 to 6 or even up to 10. When there is no external input of chlorides, as in the case of a boiler for instance, C is far higher (approximately 100 in a PWR power station).
recycling with water deterioration
This will always involve water for use in an application that creates pollution and that results in foreign compounds being included in the make-up water. This utilisation can be:
- associated with a cooling application:
- gas scrubbing with HCℓ present (household waste incineration);
- gas scrubbing with SO2 present (boiler fumes);
- gas scrubbing with HF, HCN present (blast furnace gases);
- steel decarbonising and spraying rolling mills where oil and scale will be carried away;
- nitrogenous fertiliser granulation with ammonia nitrogen solubilisation;
- quenching stations and transporting of slurry, with sulphur compound solubilisation.
- without cooling function:
- flushing during galvanoplasty with injection of soluble salts;
- gas scrubbing in the phosphate industry;
- transporting raw materials with the injection of suspended solids and of salts (various washing plants, hydrometallurgy).
The salt concentration level is no longer caused solely by evaporation and, in practice, often becomes difficult to quantify when chlorides are injected externally. Also, as the humidity condenses, it may be difficult to quantify the make-up water required (wet gas scrubbing).
Recycling rate R then becomes the only parameter suitable for estimating the make-up water usage rate.
Depending on the extent of the pollution introduced into the system, this pollution will need to be deconcentrated by a purification unit mounted on the circuit or on a by-pass (figure 11).
When this pollution is significant (gas washing water), make-up water salinity and impurities constitute a secondary parameter and, therefore, requires no primary purification. The problem to be resolved then becomes far closer to that affecting recycled industrial effluents.
water re-use or cascade utilisation
When water is rarely available, recycling may not be a method of saving enough water. Water re-use is another means that consists in using water for two successive and different tasks with, if applicable, an intermediate recovery or treatment phase.

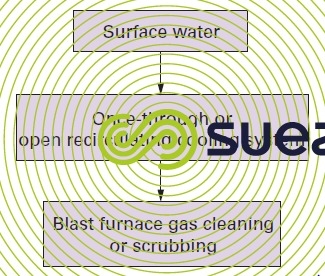

The second utilisation tends to be less "noble" than the first and, in such cases, intermediate treatment can be superfluous. For instance, the oxygen blowdown from an open recirculating steelworks gas circuit can be fed direct to the blast furnace gas scrubbing system (figure 13).
Similarly, in arid countries, more or less processed urban wastewater is increasingly being used as industrial make-up water.
selecting water sources
Apart from the financial aspect, whenever possible, the following selection criteria should apply:
- water/utilisation compatibility: carbonate balance, total hardness, temperature and, in the event of concentration, SO42–, SiO2, Ca2+, Cℓ–…;
- water compatibility with certain types of treatments intended (membranes, ion exchangers).
Table 14 puts forward a selection of water sources available depending on their intended usage.

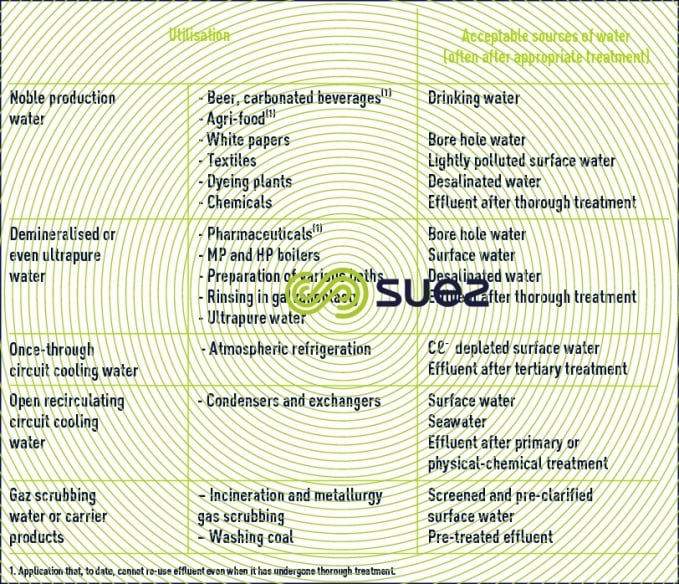

Seawater can be used in the two following applications without reducing its salinity:
- condenser cooling;
- secondary "offshore" recovery.
Seawater requires desalination in all other cases.
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












