water and cell metabolism
Reading time:It is in the external environment where they live that organisms search for the essential substances, also called essential metabolites, that they require to maintain their activities, their growth, and even their reproduction.
Water is not only essential for all living beings, in whom it is the greatest component, it also constitutes an environment which is particularly favourable for the dissemination of food and for food chains to function. The following section mainly concerns aquatic life.
metabolism concepts
Metabolism comprises all the biochemical and energy transformations (accompanied by absorption and excretion phenomena) that allow living beings to exist. All the reactions in question are catalysed by enzymes (special complex proteins) and fall into two categories:
- synthesis metabolism or anabolism, which is endoenergetic (involving energy consumption), enabling the organism to build up its substance (particularly its structural, reserve, or enzyme catalysis proteins);
- energy metabolism or catabolism, which produces the energy required for anabolism via exoenergetic reactions which break down food or reserve substances (particularly carbohydrates) which are rich in potential chemical energy, with ATP playing a role in transporting the energy; the most important process is a set of oxidation reactions which consist of the dehydrogenation of organic compounds and which are subdivided depending on the nature of the hydrogen acceptor brought into play: respiration that is aerobic (features the presence of free oxygen, and which accepts H to provide water) or anoxic (without free O2 but with the presence of oxygen mineral bonds such as NO3 or SO4, which undergo a chemical reduction by losing their O); or anaerobic fermentation (the breakdown of organic compounds in a medium without either O2 or an oxygen mineral bond).
the nutrition of living beings
Depending on the way the living beings feed themselves, a distinction may be drawn between two fundamental groups: autotrophes and heterotrophes (see figure 7).

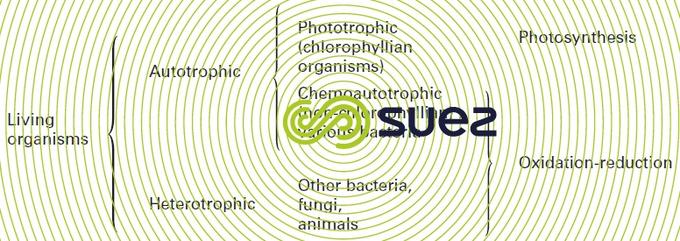

autotrophy
Autotrophic organisms are capable of synthesising their essential metabolites by assimilating inorganic carbon (CO2, HCO3-) and even methane so they can build up their carbohydrates whilst adding certain mineral salts to them: ammonia nitrogen or nitric nitrogen (for synthesising amino acids, proteins, etc.), phosphates (components of DNA and ATP ), trace elements, etc. They are the source of the natural organic material to be found in water, hence their name primary producers; there are two possible sources of the energy required for this assimilation:
- solar energy, used in the chlorophyllian pigments of phototropes (algae, aquatic plants, some rare forms of photosynthesis bacteria); the complex reactions of this chlorophyllianassimilation, which is called photosynthesis, are symbolised by the formation of a glucose molecule:

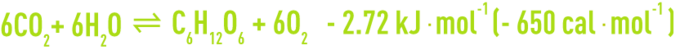
This biological process is the most important one in the natural world in quantitative terms; moreover, practically all the other living beings and traditional energy sources draw from it, and it also forms the main source of oxygen in water and in our atmosphere.
The above equilibrium can be reversed: from right to left, it is an aerobic respiration reaction, as we saw above.
- chemical energy, from a redox reaction based on minerals in the case of chemotropic bacteria: many of them are important in terms of water treatment, particularly:
- nitrifiers: those belonging to the Nitrosomonas genus, which oxidise ammonium into nitrites, those belonging to the Nitrobacter genus, which transform nitrites into nitrates;
- certain iron and/or manganese bacteria, which oxidise ferrous and manganous ions into manganic and ferric oxides/hydroxides;
- sulphur-oxidising bacteria (also known as sulphur bacteria) which oxidise reduced forms of sulphur (particularly H2S) into colloidal sulphur (the Beggiatoa-Thiothrix group) or into sulphuric acid (the Thiobacillus group).
These bacteria are generally aerobic, apart from a few exceptions which live in anaerobic environments (certain acetogenic and methanogenic bacteria involved in methanisation, for example).
heterotrophy
Heterotrophic beings can only feed off organic material that has already been built up (by autotrophes or other heterotrophes, hence the food chain concept).
During catabolism, this substrate is broken into simpler molecules which are then oxidised to supply the energy required for anabolism: the two phenomena are therefore closely linked and entail coupled oxidation-reduction reactions; the organic substrate is used both as a source of energy in catabolism and as a supply of cell components in anabolism.
Heterotrophic organisms include all non-chlorophyllian beings: bacteria (except chemotropic bacteria), fungi, and animals. The end of this sub-chapter will mainly be devoted to bacteria.
The enzymes required for metabolism can be either inside the cell, or excreted into the outside environment in order to cut up molecules that are too long so they can be disseminated through the cell wall.
Depending on the type of respiration or fermentation carried out (see above), the hydrogen acceptor required for catabolism’s oxidation reactions is either free oxygen in an aerobic medium (for example: the bacteria in activated sludge), or, in an anoxic medium, the bonded oxygen in mineral compounds such as sulphates (reduced in terms of H2S and sulphides by sulphate-reducing bacteria) or nitrates (reduced in terms of diatomic nitrogen by denitrifying bacteria), or an organic compound in an anaerobic environment (for example: methanisation bacteria).
The ultimate reaction products are normally CO2 and H2O when working in the aerobic phase, and CO2 and CH4 when working in the anaerobic phase. Inbetween aerobic and anaerobic bacteria strictly speaking, there are semi-anaerobic bacteria: their catabolism depends on the medium’s physical and chemical conditions.
practical consequences
We shall see the application of these various concepts in Chapter what water should we treat ? and why ? (nitrogen and sulphur cycles) and in the chapters dealing with biological treatments for drinking water and municipal and industrial effluent. For example, these enable an understanding of why the heterotrophic bacteria in a denitrification treatment require an organic nutrient, although none is required for the autotrophic bacteria involved in nitrification; in the first case, nitrates supply the oxygen used in respiration, while in the second case, NH4 oxidation (by oxygen supplied from outside) provides the energy required for the chemosynthesis of essential metabolites.
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












