the three states of water
Reading time:Water’s structure depends on its physical state.
Its gaseous state (vapor) precisely matches the H2O formula and particularly the angular model (figure 1).

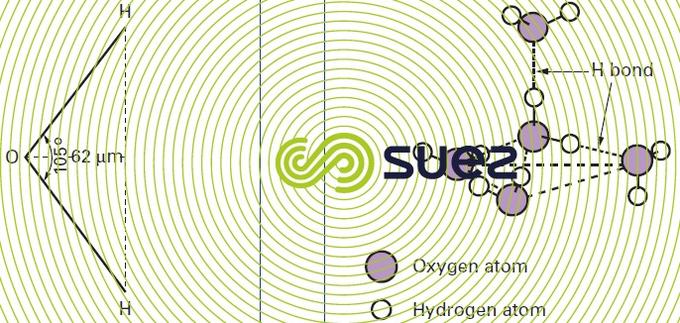

However its condensed states (water and ice) are more complicated, and this complication is the reason for their atypical properties (see physical properties)
In its solid state (ice), the basic arrangement consists of a central water molecule and four peripheral ones, with the whole assembly forming a tetrahedron (figure 2). This structure is due to the association of molecules under the influence of intermolecular bonds called hydrogen bonds: each hydrogen atom in a water molecule is attracted by the oxygen atom (which has an opposite polarity) in the neighbouring molecule. Depending on the type of ice, basic tetrahedrons form various crystalline structures, the most common of which is a hexagonal shape (ordinary ice). This symmetry is found in snow crystals in the form of a large variety of hexagonal star shapes (Photo 2).
Study of crystallographic variations using the Raman spectrum in particular, allows the shift from a liquid state based on the ice’s hollow structure to be understood: as the temperature increases, the “hollows” are gradually filled in by interstitial water molecules, until the crystalline structure completely collapses upon fusion; in liquid water, the tetrahedral-based structural model is still similar to the one ice has, except that the small aggregates of bonded molecules are mixed with free molecules, which gradually replace them as the temperature increases until boiling point is reached, when all the water molecules become free in their vapor phase, as referred to above.



Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












