terms used by water analysts
Reading time:In order to treat water, you have to understand it and therefore be able to characterize it as precisely as possible. Moreover, in the vocabulary used by water specialists, certain terms differ substantially from normal scientific terminology used.
The parameters listed below are the most common ones. They correspond, either directly or indirectly, to concentrations. Refer to water analysis and treatability orchemistry and reagents to learn about the analysis procedures required for determining these parameters.
turbidity
In relation to measuring suspended solids, the turbidity value gives an initial indication of the colloidal materials content (whether of mineral or organic origin) disturbing the water (this concept is therefore the opposite of clearness). It is assessed either by measuring the visibility limit for a defined object (a platinum wire, a Secchi disk), or more scientifically by measuring the light diffused at an angle of 90° in relation to the incident light (see laboratory methods and recap table) by devices called turbidimeters, which are themselves calibrated using opalescent control suspensions (formazine) the results of which are expressed in NTU or NFU, depending on the operating conditions.
suspended solids (SS)
This is the concentration of the dried part (minus water content) of all the suspended elements in water which are large enough to be retained in a filter with a given level of porosity, or collected in the form of a centrifugation pellet. There is no general relationship between turbidity and suspended solids, but such a correlation can be established empirically for each type of water.
fouling index
This represents the water’s fouling power. It therefore also has a relationship with suspended solids and is a factor in treatments that use membranes (see measuring global parameters)
colour
More often than not, the true colour after filtration is due to the presence of dissolved or colloidal organic materials. There is not always a relationship between the colour and the organic materials concentration. It is measured by comparison with a reference solution (Platinum - Cobalt) for which the concentration unit, expressed in mg · L-1, is also called a Hazen degree.
mass concentration in relation to a liquid volume
This is the mass of the substance dissolved or dispersed in a given volume of water. It is expressed in mg · L-1, g · m-3, g · L-1 …
ppm concentration
A ppm is one part of the substance in a solution, mix or suspension per million parts of another substance (water in the case at hand). Strictly speaking, this term should only be applied to relationships between masses or between volumes (for example: mg · kg-1), but ppm is still used in the practice of water treatment as a concentration unit equivalent to 1 g · m-3 or 1 mg · L-1, with the density of water (for pure water mv = 1 kg · L-1) allowing this approximation to be made with only a negligible error.
gramme-equivalent
The gramme-equivalent is the molecular weight quotient for a substance times the number of charges with the same sign carried by the ions that a molecule of this substance releases in an aqueous solution.
Thus, a molecule of orthophosphoric acid, H3PO4, releases three positive charges (3H+) and three negative charges (PO43-). A gramme-equivalent of H3PO4 therefore equals 1/3 of the weight of one mole of H3PO4.
normality
A normal solution is one that contains one gramme-equivalent of the substance in question per litre of solution. Multiples and sub-multiples of the normal solution are also used (10N - N - N/10 solutions, etc.).
Generally speaking, when you make an electrolyte with normality N2 act on a volume V1 for an electrolyte with normality N1, the volume V2 is deduced from the relationship:


milliequivalent per litre
The milliequivalent per litre (meq · L-1), which is the concentration of a solution N/1000, is often used in practice.
french degree
This is still used in France to express the concentrations of the main ions in water and is the concentration of a solution N/5,000.

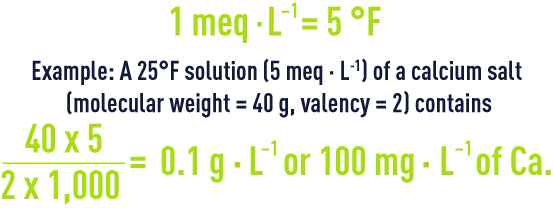
correlating the various types of degrees
The table 10 below (to be read horizontally) provides the coefficients for converting the various units still in use.



titration for hardness (TH)
The titration for hardness, also known simply as the “hardness”, represents the concentration of alkaline-earth ions in water. A distinction is drawn between:
- Total TH: Ca and Mg content;
- Calcium TH: Ca content;
- Carbonate hardness: the hydrogen carbonate and carbonate content of Ca and Mg. It equals the m-alk (see below), if the TH is greater than the m-alk, or the TH, if the m-alk is greater than the TH;
- non-carbonate hardness (permanent hardness): indicates the Ca and Mg content with strong anions. It is equal to the difference between the total TH and the bicarbonate hardness.
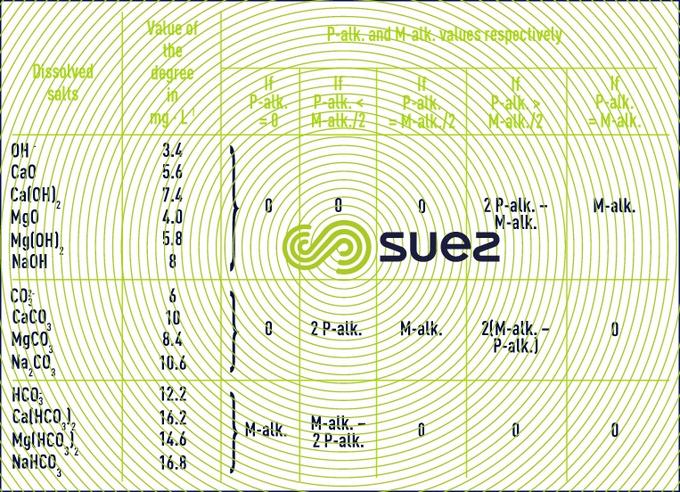
phenolphthalein alkalinity (p-alk) and methyl orange alkalinity (m-alk)
The relative values for p-alk and m-alk allow water’s content in terms of hydroxides, carbonates and alkaline and alkaline-earth hydrogen carbonates to be known. The table 11 shows that:
- the p-alk allows the hydroxides content as a whole to be determined, and only half of the carbonates content;
- basic reacting compounds (hydroxides, carbonates, composés à réaction basique (hydroxydes, carbonates…) up to a pH of approximately 4.5, so also weak acids. For natural water, it mainly namely represents the hydrogen carbonate ion HCO3- (also known as bicarbonate).
In some highly-polluted water (wastewater), the m-alk also covers weak organic acids (acetic acid matières, etc.).
measurement of salts of strong acids (SSA)
In natural water, there is no strong free acid; only salts of these acids are present, particularly sulphides, chlorides and nitrates. The salts of strong acids measurement expresses the overall salts content of these strong acids.
salinity
Water’s total salinity is the sum of the cations and anions present, expressed in mg · L-1.
The dry solids content obtained by evaporation is generally lower due to the breakdown of the hydrogen carbonates into carbonates and CO2.


permanganate value
All the substances capable of being oxidised by potassium permanganate (KMnO4) are grouped together under this term. Basically they are organic materials, and sometimes mineral reducing agents. This measurement is mainly performed on natural water but is tending to be progressively replaced by organic carbon measurement. There are various methods (depending on the temperature, the reaction medium’s pH, and the contact time) which give different results. The most common measurement is now a high temperature measurement in an acidic environment with a value expressed in O2 concentration Outside France, this measurement is also called the “permanganate demand”, and even the “COD”, which is not to be confused with the following measurement.
chemical oxygen demand (COD)
COD, which is used in particular for effluents, is the overall hot consumption of the oxygen in potassium dichromate and is representative of the greater part of organic compounds, as well as of oxidizable mineral salts.
biochemical oxygen demand (BOD)
This is the quantity of oxygen consumed at a temperature of 20°C and in darkness over a given time in order to ensure the oxidation of organic materials in water using biological techniques. Conventionally, BOD5 is used, or in other words the quantity of oxygen consumed after five days of incubation. BOD5 is only representative of biodegradable organic carbon pollution if you make the effort to block nitrification reactions when measuring.
total organic carbon (TOC)
This represents the carbon content linked to the organic material, and is based on measuring the CO2 after full oxidation.
This measurement is quick and only requires a small volume of sample, but measuring apparatus is costly. It is widely used in the drinking water domain and is progressively replacing permanganate oxidizability. The correlation between the two measures is to the order of 1 on natural water. On the other hand, these two parameters are difficult to correlate with those used in the wastewater domain owing to the difference in the nature of OM.
kjeldahl nitrogen (TKN)
TKN groups together nitrogen that is present in organic form and nitrogen in ammonia form. It is sometimes incorrectly called total nitrogen
global nitrogen (NGL) or total nitrogen (TN)
This term covers all the nitrogenous forms present in water, or in other words organic nitrogen, ammonia nitrogen, nitrite and nitrate. It is therefore an extension to the term TKN, encompassing oxidised forms of nitrogen. Reference is also made to inorganic nitrogen (see German standards);


measurements of 2-hour clarified effluents
Sometimes in France the suffix ad2 is used for the COD, SS, BOD5, NH4 and other parameters. For example, COD ad2 means that the COD measurement was carried out on a clarified sample over a two-hour period, and which therefore did not have any large suspended solids.
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












