laboratory methods and recap table
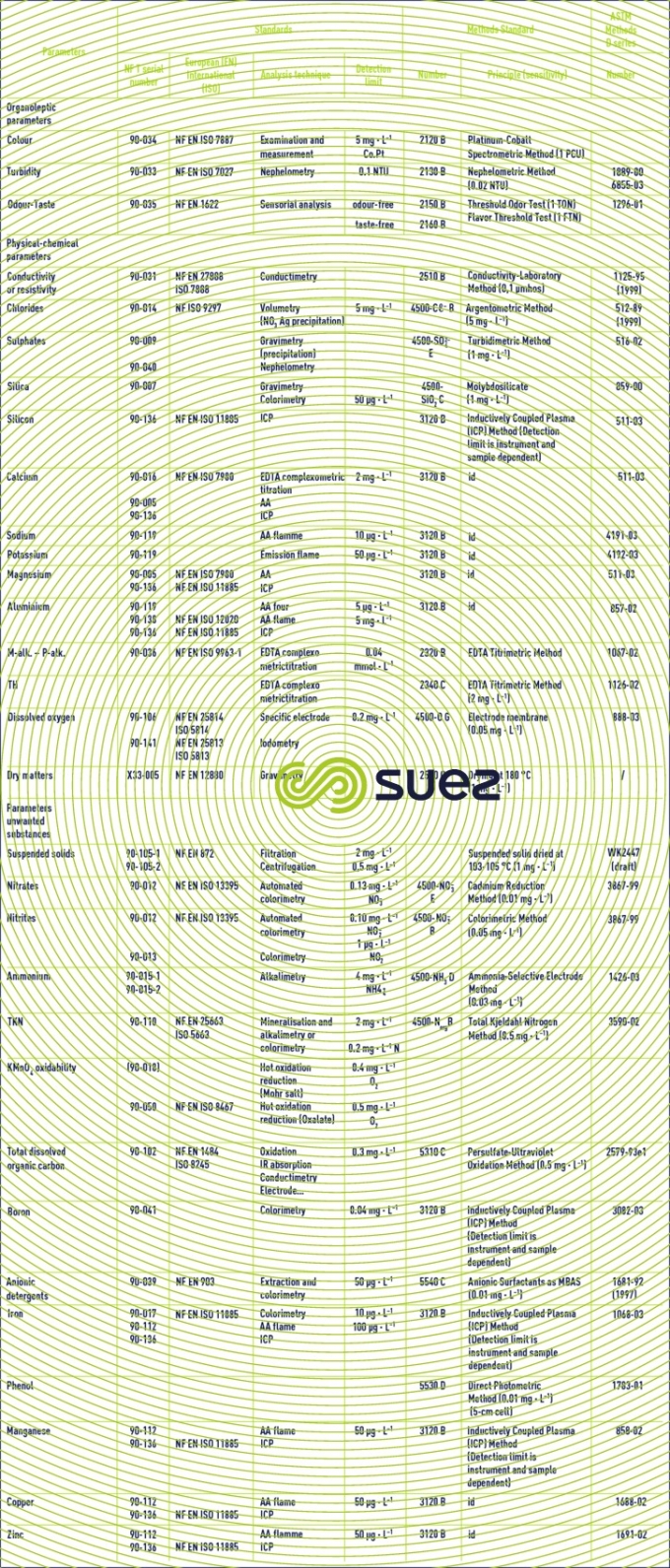
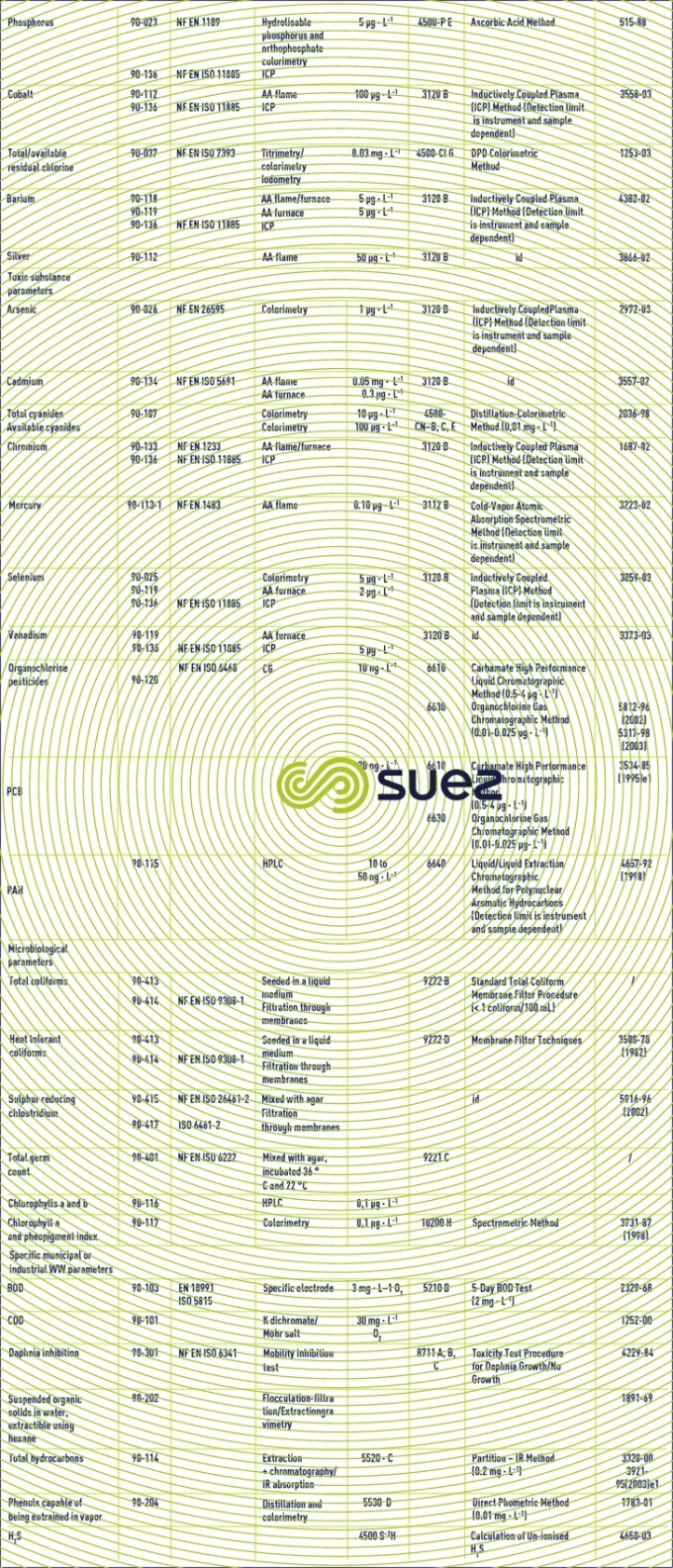
Reading time:Table 2 gathers the main analysis techniques used in water treatment applications together with the French (AFNOR), European (EN), international (ISO) or American (Standard Methods or ASTM ) standards used to define these techniques.
There are other standards (German DIN, Russian GOST …).
preliminary concentration
Methods based on concentration by extraction are mainly used when analysing organic micro pollutants. There are four techniques:
- liquid-liquid extraction and concentration of the solvent. Dichloromethane is the option frequently selected for subsequent determination of pesticides and PAH:
- extracting volatile compounds using the closed loop stripping technique (CLSA);
- extracting volatile and semi-volatile compounds using the simultaneous distillation extraction (SDE) technique;
- cryometry.
taste evaluation
To better evaluate of the taste in drinking waters, water tasting techniques are increasingly being used for research and control purposes. Two of these techniques deserve further comments:
- taste threshold determination (AFNOR NFT 90.035): taste-less water is used to dilute the water to be tested. The tasting session begins with the most diluted samples, gradually increasing the taste water concentration until taste is detected. The threshold is the dilution level that should be perceived by most operators (panel of at least three tasters):
- determining the "taste" profile. This method provides more information than the previous method. A group (panel) of trained and experienced tasters (minimum of four) establishes an odour, taste and sensation profile. Each of these parameters is scored according to an intensity scale of 1 to 12. Their nature must be identified from a list of descriptors.




gravimetry
The gravimetry principle is based on measuring of a mass that is equal or directly proportional to the concentration of the element to analyse. An example of a gravimetry application is the measurement of suspended solids after solid-liquid separation. SO42– ions can be determined by precipitating BaSO4…
Clearly, these methods are limited by the precision of the balance equipment used.
volumetry
By definition, this method consists in measuring a volume of a titrated solution that is proportional to the concentration of the target element. The following reactions may apply:
acid base neutralisation
When determining P-alk. and M-alk. for instance, the titrated acid used is sulphuric acid. Colored indicators have been selected according to the pH at which they undergo a color change.
precipitation
As an example silver chloride precipitates using a titrated silver nitrate solution. The end of the reaction is signaled by the reddish brown color of the silver chromate precipitate, a salt that is more soluble than silver chloride.
oxidation reduction
In the cases of permanganate oxidability and COD , potassium permanganate and potassium dichromate oxidants are injected in excess in relation to the reducing organic matter that requires titration. A titrated reducing agent (Mohr salt, for instance) is used to measure the remaining oxidant. Once again, the end of the reaction is signaled by a color change.
complexometric titration
TH is determined bycomplexing Ca2+ and Mg2+ ions with a titrated solution of filtered EDTA complexing agent titrated solution. The end of the reaction is also colorimetric.
nephelometry
Turbidity measurement (see Terms used by water analysts and various other units) uses this principle illustrated in figure 2 below. Particles contained in water will diffuse light to a greater or lesser extent. When an observer is positioned at an angle of 90° in relation to the incident light, he receives a diffused light with an intensity that is proportional to the particle concentration present in the water.
Some apparatus compare diffused light with transmitted light to measure turbidity for medium that do not diffuse much light.

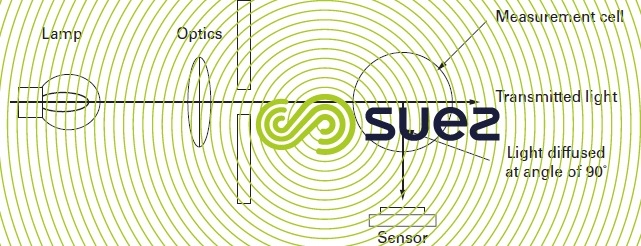

In European countries covered by the standard ISO 7027, the measurement must be carried out in infrared light at 860 ± 60 nm and when:
- turbidity is below 40, diffused light intensity is measured at 90° (results expressed in NFU units);
- turbidity is above 40, transmitted light is measured (results expressed in FAU units).
The ASTM standard continues to use visible light and results are expressed in NTU.
Note: in all cases, calibration is carried out using the same formazine suspensions.
Turbidity measurement calls for caution: avoid air bubbles; prepare dilutions for high turbidity conditions. The same apparatus must be used for comparative turbidity measurements.
amperometry
Oxidants such as chlorine, chlorine dioxide or ozone can be analysed using amperometry. A depolarisation current between two electrodes is proportional to the oxidant’s concentration. Amperometry allows continuous measurements to be carried out on the field. To improve laboratory precision, a reducing agent (phenylarsene oxide) is injected to lower the depolarisation current to a plateau that becomes characteristic of the end of the reaction. In effect, this method is volumetric but with the end of the reaction is detected by amperometry.
ionometry
The potential difference measured between a specific electrode and a reference electrode is a logarithmic function (Nernst’s law) of the target element activity. The pH measurement electrode, a glass membrane electrode, remains the best version of a specific electrode. Ionometry is also used to measure oxidation reduction potential ( ORP ).
By means of a set of specific electrodes and an ionometer (high performance pHmeter), the ionometric system can be used to measure some fifteen different ions. Additionally, coupling specific electrodes such as indicator electrodes in a potentiometric measurement provides very accurate results.
The most widely found electrodes are those used to measure free F–, CN–, S2– ions. Additionally, specific electrodes are very useful to analyse elements in the field. However they require the test water to be kept at a constant temperature and the medium to be subjected to a given ionic force.
spectrophotometry
molecular absorption spectrophotometry
This is the most widely used water analysis method. As a preliminary operation, the element targeted must undergo a specific color reaction. This method is based on the fact that any coloured solution crossed by a beam of light will only let a fraction of the incident light through; the amount of light absorbed will be proportional to the concentration of the target colored compound (Beer-Lambert law). This technique has been used to develop continuous flow, laboratory analysis systems, the industrial use of photocolorimetrics for the "continuous sequential" measurement of a range of parameters (silica, ammonium ….).
UV and IR absorption spectrophotometry
In the water industry, these techniques are mainly used to quantify OM groups.
The measurement of the UV absorption at 254 nm is an index that is characteristic of substances that have one or more double chemical bonds.
The same measurement carried out at other wavelengths completes the examination (example of humic acids).
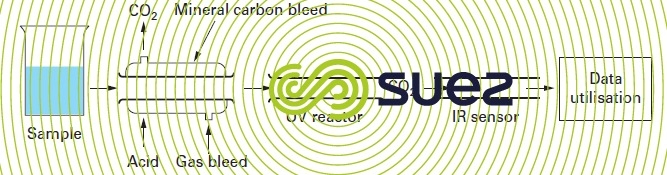
Measuring the TOC (NF EN 1484) involves mineralising organic carbon through chemical oxidation and UV (figure 3) or through combustion, coupled with IR detection of CO2. The method detection limit is 0. 2 mg · L-1 with a 10% accuracy.


The CH2 index is used to measure pollution generated by hydrocarbons. Typically, a technique based on the absorption of – CH, – CH2, – CH3 bonds in the infrared spectrum ranging from 2 800 to 3 000 nm is used. Numerous operating methods exist, however, their scope remains uncertain and their interpretation difficult.
atomic absorption ( AA ) spectrophotometry
Plasma atoms obtained through thermal or electrical excitation can absorb the radiation from discrete and specific wavelengths.
In atomic absorption with flame techniques (air/acetylene or nitrous oxide/acetylene), the water sample containing the target metal elements is dispersed into a very hot flame as a mist of fine droplets. At high temperatures, the chemical bonds are destroyed and the metals that are released to form a plasma of free atoms. This atomic mist is illuminated by a wavelength that is characteristic of the parameter analysed. The amount of light absorbed by the plasma is proportional to the element concentration.
Smaller volumes are used with the electrical atomisation flame-free technique. The misting mechanism consists of a graphite tube heated to a temperature of between 1,500 and 2,800 °C.
flame emission spectrophotometry
A breakdown and dissociation of metal traces in the atomic state occurs when a water solution containing metals is misted into a flame. The flame thermally excites the metal atoms and their return to a fundamental state is accompanied by the emission of radiation having a wavelength specific to the target element with an intensity directly proportional to concentration. This technique is suitable for the direct measurement of base elements such as: Na, K, Li.
inductive coupling plasma (ICP) spectrophotometry
Inductive coupling plasma spectrophotometry is a technique based on atom emission phenomena where an argon plasma constitutes the source of atoms. At high temperatures, a mixture of atoms and charged particles forms within the argon gas as follows:


A high frequency generator produces the plasma through induction.
Its temperature varies from 6,000 to 8,000 °C. The target elements are injected into the plasma and converted into atomic vapor and, if applicable, into ionic vapor through excitation as they collide with the elements that form the plasma.
This technique has a wider range of utilisation than the flame-free atomic absorption method but suffers from a lower detection capability. The plasma high temperature is used to limit matrix interference and, therefore, the ICP can be widely used to search for heavy metals in municipal WW plant sludge where the matrices are extremely rich in organic matter and require mineralisation in an acid medium.
All spectrophotometers are equipped with a light dispersion system used to select the appropriate wavelength and a photomultiplier to amplify the received intensity.

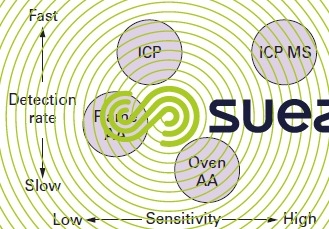

fluorescence
Fluorescence is a luminescence phenomenon: molecules emit radiation in all directions due to energy received from an incident light source. Fluorescence is the property of aromatic cyclic compounds.
It is measured using spectrofluorimeters and an incident UV light with a 90° readout of UV and visible light.
chromatography
As a rule, to identify and measure OM, we use chromatographic techniques.
In gas phase chromatography ( GC ), the capillary column technique is used because of its unrivalled resolution capability, the availability of universal detectors and the ease with which it can be combined with mass spectrometry (SM in French).
A liquid or gas phase chromatograph comprises three sections: an injector, a splitter column and a detector. The sample is first injected using a micro syringe and the molecules split in the selective adsorption column according to an eluant concentration or a temperature gradient.
When the separated compounds emerge from the splitter column, the separated components successively pass through a detector whose purpose is to emit a signal. The intensity of this signal is proportional to the amount of compound injected. By virtue of calibration, this signal provides a quantitative analysis (example of a chromatogram: Figure 5). Universal detectors have an average sensitivity level to most organic compounds whereas “specific” detectors provide a far more intense response for some chemical groups.

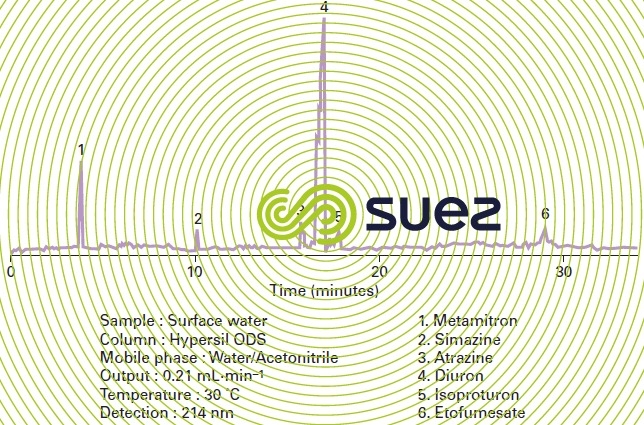

The most common detector is the flame ionisation detector (FID). The specific detectors consist of the electron capture detector (ECD) that is sensitive to halogen compounds, thermo-ionic detectors for nitrogen and phosphorus compounds and finally the photo-ionisation detector (PID) for aromatic compounds.
High pressure liquid chromatography ( HPLC ) uses aqueous or organic solvents. This system comprises many more analytical techniques than the gas phase variant. Using a polar liquid phase to elute a column containing an apolar phase or reverse phase chromatography is used to determine PAHs.
Ionic chromatography (through ion exchange) is used to separate large number of cations and anions.
Exclusion chromatography separates compounds according to size over a porous gel. It is used to determine apparent molar weight and therefore fractions of different molar weights are available for additional analysis.
polarography
Polarography is based on the analysis of current intensity-potential curves. Between two electrodes (one that is usually mercury drop polarised and the second which is the reference electrode) current intensity is recorded for a continuous variation of potential. The intensity differential between the two levels is proportional to the oxidised or reduced elements. One of this main applications for this method is the analysis of metal cations and their “speciations” (degrees of oxidation, sequestering). Variants of this technique have enhanced sensitivity.
mass spectrometry ( MS )
The combination of GC-MS allows measurement of the various groups of compounds that are ‘chromatographable’ in the gas phase by using one single extraction solvent (e.g. dichloromethane) and one single chromatographic splitting operation.
Electrons bombard the compounds eluted by the chromatograph, thus fragmenting these compounds. All the ions detected (relative quantities and their atomic masses are measured) form the molecule characteristic spectrum. The computer helps the technician to apply the spectra and to identify the molecules involved and will do this even when splitting within the column has not taken place satisfactorily, hence the advantage of the mass spectrometer over specific detectors (figure 6).

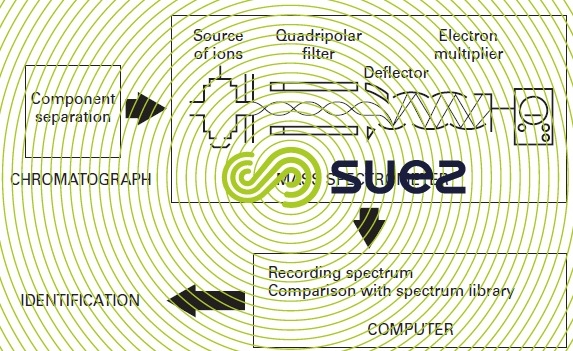

measuring radioactivity
When inspecting water from the mains distribution network, determination is normally carried out without prior chemical separation. The following is evaluated
- global activity α;
- global activity β;
- spectrometry Ɣ;
In the more complex cases, detailed radioanalysis are carried out following chemical separation. When checking water, only activities b and g are taken into consideration for monitoring changes in radioactivity. However, water radioactivity is always low and, therefore, only a few apparatus are capable of producing satisfactory results. The most frequently used detectors are:
- ionisation gas meters (Geigy-Muller counter, proportional counter);
- radiation-sensitive scintillation or semiconductor detectors
combined techniques
A large number of analytical determinations involve several techniques. One example is the GC-MS combination: splitting followed by detection.
Also, general methods for the determination of the OM can be divided into two sections: reaction and detection (table 3).



Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












