specific analysis
Reading time:biological oxygen demand ( BOD )
The biochemical oxygen demand is normally measured after five days. This is the BOD5; it refers to the adaptation and bacterial synthesis phases. It may be worthwhile knowing the ultimate BOD, normally measured after 21 days, which includes the auto-oxidation phase (endogenous metabolism).
dilution method (NF EN 1899-1)
Appropriate solutions are prepared using the test water and a pure seed water, checking from time to time that the latter is not absorbing noticeable amounts of oxygen. Seeding will not be required when dealing with municipal wastewaters. The best results are obtained when the oxygen loss obtained during the test ranges from 35 to 60 % of the initial content. The dilutions used are based on the test water pollution content. In order to establish these dilutions the COD can be measured considering that the BOD5 is lower than the COD and that the COD and that the COD / BOD5 ratio typically ranges from 1.5 to 3 for municipal wastewaters. Assuming that saturated water at 20 °C contains approximately 8 mg· L-1 of oxygen one can write:

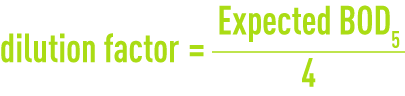
At least three different dilutions should be prepared to surrounded the pre- sumed value. Dilutions are kept in the dark for five days at 20 °C. The dilution water used should remain at that temperature and in perfect balance with the atmosphere. This is achieved by keeping the dilution stock in a controlled oven or in a thermostatically controlled bath used to incubate diluted samples.
In order to avoid the effect of positive interference on the result in the event of nitrification, nitrifying organisms should be neutralised by adding thiosinamine (allylthiourea) to the samples at the time of analysis. The dilution water should be reseeded before resuming the incubation process.
The BOD5 measurement being a biological measurement it is clear that the presence of microorganisms capable of degrading the pollution matter should be ensured. For seeding purposes one can use the following:
- municipal wastewater with a COD lower than 300 mg O2 · L–1;
- clarified effluent produced by a wastewater treatment plant;
- water taken downstream of the point at which the water to be analysed is discharged;
- water containing microorganisms that are adapted to the test water (case of industrial effluents containing substances that are difficult to biodegrade).
manometric methods
Apparatus based on manometric measurements can be used to monitor in a sealed reactor how oxygen disappears from the gas phase with time. This method also requires nitrifying organisms to be neutralised.
When deciding on a treatment process, it is often useful to know the following:
- total BOD5 measured on the raw water sample;
- dissolved BOD5 measured on the sample filtered through a membrane;
- BOD5 after clarification (in French, AD), that includes colloidal and dissolved BOD ; in France, it is quite usual to carry out this clarification within two hours.
The uncertainty related to BOD5 measurements can be significant, in particular in the case of raw industrial WW when the seeding used is not appropriate. With raw municipal WW , the uncertainty should not exceed 10 %; however, in some cases the uncertainty may be as high as 50 % following thorough biological purification (BOD 5 < 5).
chemical oxygen demand (COD)
The standardised method (NF T 90.101) applicable to municipal wastewaters, uses potassium dichromate in a hot sulphuric medium, a powerful oxidant whose consumption during the procedure results in the measurement of the COD. The uncertainty of this analytical procedure is around 10 % for levels above 30 mg · L–1 of O2. Alternate methods are more appropriate for lower CODs.
The COD represents everything that can be oxidised including some mineral salts suitable to oxidation such as (sulphides, sulphites…) and most of the organic compounds. Only a few nitrogen and hydrocarbons compounds will escape this powerful oxidation.
The representative nature of the COD is no longer satisfactory when chloride contents increases above 2 g · L–1 (the chloride ion precipitates the oxidation catalyst required by the reaction). In this case the proportion of mercury sulphate (II) needs to be increased to act as a chelating agent (forming chloromercurate (II) that is soluble but not oxidable). For high chloride content the best solution consists in diluting the sample to reduce the interference. However, care must be taken not to go lower than the method detection limit.
Potassium permanganate oxidability either cold (4 hours) or hot (10 minutes boiling), can be used as a field procedure to monitor possible changes with the characteristics of the water at different locations of a WWTP (especially purified water) or with a drinking water source.
When measuring the TOC (see laboratory methods and recap table), the oxidation of organic matter should normally be more complete than when measuring the COD .
It is often beneficial to measure the three TOC , COD and BOD5 parameters as the COD / TOC and COD / BOD5 ratios can be indicative of a specific pollution (industrial effluents).
suspended solids
The membrane filtration method appears to be the easiest, however, the protocol as detailed in standard NF EN 872 must be carefully followed to obtain reliable results. There are many sources of error: membrane type, vacuum level, acceleration level ( NF T 90.105-2 centrifugation method), samplers capacity, rinsing method used after separation and especially post precipitation between sampling and analysis (hydroxides, carbonates, phosphorus, gypsum…).
volume of settleable matter
The analysis is carried out on samples that have been screened through a 5 mm strainer in order to remove bulky debris. Settleable applies to matter that is deposited in a liquid at rest over a period of two hours by standards. The test tubes used are graduated and conical or cylindrical-conical, therefore easing the measure of the settled volume.
hydrocarbons
Many analytical methods exist to assess the presence of hydrocarbons and these methods can produce a wide variety of results. There are many parameters involved: solvents, extraction conditions, gravimetry or IR absorption, IR absorbance calculations (calibration method, wavelengths used). The methods used must always be specified and the operating methods carefully followed. Thus, results obtained according to standards NF T 90.114, and 90.203 can differ widely.
The NF T 90.202 method is normally used to measure oil and fats present in municipal WW or in effluents from the food industry. This is the method used to measure organic suspended matter in waters that were previously extracted with hexane.


nitrogen
To follow the evolution of nitrogen throughout a treatment strain one needs to know its different forms:
- ammonia nitrogen;
- nitrate nitrogen useful to detect any possible biological limitations;
- nitrite nitrogen;
- Kjeldahl nitrogen ( TKN ) the sum of ammonia nitrogen and organic nitrogen. Therefore, this form does not include oxidised nitrogen compounds: nitrites, nitrates and some nitrate organic compounds. Measuring low TKN contents is a delicate operation.
It should be noted that in France the sum of all nitrogen forms is named global nitrogen (NGL) versus total nitrogen TN in other countries. All analysis must be carried out after stopping any biological reactions at the time of sampling.
phosphorus
The analysis is used to distinguish three different forms of dissolved phosphorus:
- orthophosphates;
- polyphosphates (after acid hydrolysis);
- organophosphates (after acid hydrolysis with oxidation).
Inductive coupling plasma spectrometry allows to directly measure total phosphorus.
sulphur
Sulphides can be determined by iodometry after the sulphides have been fixed, by argentimetry with potentiometric monitoring using a silver indicator electrode, by colorimetry for low contents or by measuring the ionic activity of sulphides with a specific electrode and a reference electrode. The latter method is by far the most reliable measurements. Determining other reduced forms in industrial WW continues to be a delicate operation (thiosulphates, dithionates, sulphites, thiocyanogens…). Selective methods involving precipitation or chelation must be avoided. Ionic chromatography can be used in certain conditions.
Sulphates also need to be taken into account when monitoring the methane fermentation of some IWW.
total alkalinity (M-alk.)
This parameter should be monitored throughout the medium nitrification (acidification) or denitrification (alkalinisation) process.
heavy metals
The total concentration of heavy metals should be analysed once the sample has been mineralised. An atomic absorption spectrophotometer is used for this analysis. The most common heavy metals are: cadmium, mercury, lead, hexavalent and total chromium, copper, nickel. These are tested for in the following main reasons:
- surface treatment effluents before discharge into the receiving medium or into the drain;
- effluent before biological purification;
- sludge (standards applicable to agricultural recycling).
Additionally, there might be the need to assess the content of certain trace elements that enhance methane fermentation (e.g. nickel-cobalt).
toxicity
Toxicity is a complex concept: it encompasses the action of a great number of compounds that can be present under a wide variety of forms (chelated, ionised, oxidised…). Toxicity can only be evaluated by means of a biological test.
The most commonly used test is the one that uses daphnia (NF T 90.301). The short-term inhibition of Daphnia magna (otherwise known as daphnia) (Crustaceans, Water fleas) mobility is determined in the effluent. Results are expressed in equitox defined as follows: effluent contains one equitox per m3 when 50 % of a daphnia population is immobilised within 24 hours.
mohlman index
(see different types of sedimentation)
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












