physical properties
Reading time:The most interesting physical properties in terms of the treatment of water are as follows.
density
Due to the gradual compacting of the molecular structure, the density varies depending on the temperature and the pressure.
Pure water under normal pressure experiences a maximum at approximately 4°C (exactly 3.982°C) and its variation as a function of the temperature is as follows (table1) :

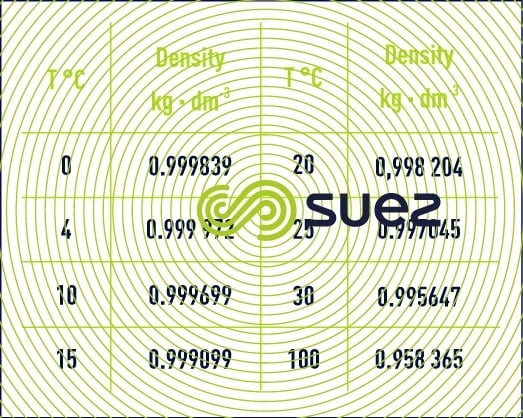

This property of water has various consequences, both in the natural world (stratification phenomena in lakes) and at treatment plants (for example: rising sludge in clarifiers).
At the pressure levels encountered in hydraulic systems, water is considered to be an incompressible fluid. As a matter of fact, it is a slightly elastic fluid: its volume decreases by approximately 0.048 % each time the pressure increases by one atmosphere.
Sea water, with a salinity of 35 g · L-1, has an average density of 1.0281 kg · L-1at 0°C; a salinity variation of 1 g · L-1 causes a variation in mass of 0.0008 kg · L-1.
Note: as pure water is the reference substance for measuring the density of liquids and solids, by definition its density equals 1.000.
thermal properties
specific heat (or specific thermal capacity)
This is very high for a liquid, being 4.18 kJ · (kg·°C)-1 or kJ · (kg . K)-1 (or 1 kcal · (kg · °C)-1 in old units) at a temperature of 20°C. It varies depending on the temperature, with a minimum of 4.1784 kJ· (kg · K)-1 at 30°C, rising to 4.2159 kJ · (kg · K)-1 at 100°C.
transformation enthalpies (or latent heats)
For fusion these are 334 kJ · kg-1 (or 6.01 kJ · mol-1) while for vaporisation the value is 2,259 kJ · kg-1 (or 40.657 kJ · mol-1) at normal pressure and at a temperature of 100°C.
The importance of specific heat and the vaporisation enthalpy ensures that the large expanses of water on the Earth’s surface constitute real heat accumulators. This is also why water is used as a heat-transfer fluid.
viscosity
This is the ability of a fluid (liquid or gas) to withstand various internal movements (turbulence for instance), or overall movements (flow for example). This resistance is due to the moving molecules’ reciprocal friction. It is the reason for losses of kinetic energy (head loss) and therefore plays a major role in water treatment.
A distinction is drawn between two types of viscosity:
- dynamic (or absolute) viscosity µ, which is defined by the relationship between tangential shearing stress T (in Pa) and speed gradient V (in m/s) in a direction y (in m) that is perpendicular to the slide plane:

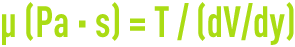
Standard sub-multiple: mPa · s




- kinematic viscosity v, which is defined by the relationship between the fluid’s dynamic viscosity µ and its density ρ:


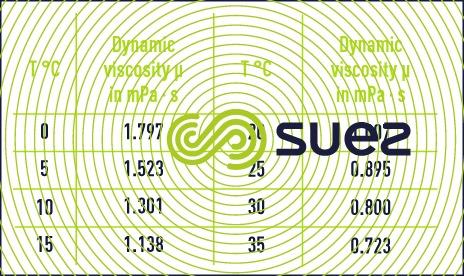
Water’s viscosity decreases when the temperature increases (table 2).
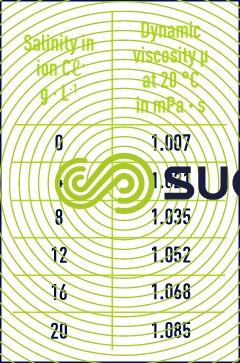
To the contrary, it increases with the content of dissolved salts; sea water is therefore more viscous than river water (table 3).
The pressure has a quite special effect on water’s absolute viscosity. Unlike other liquids, moderate pressure makes water less viscous at low temperatures: to an extent it crushes its molecular organisational structure. When the pressure continues increasing, water assumes a liquid structure free of internal stresses, and follows the general rule - in other words, the viscosity increases with the pressure level.
surface tension
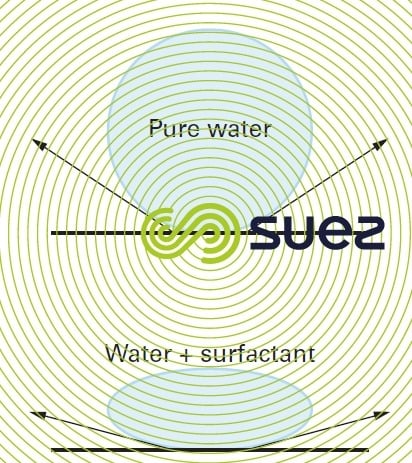
This characterises a property of interfaces (surfaces that limit two phases). It is defined as a tensile force which acts on the liquid’s surface by always tending to reduce the extent of the surface to the greatest possible extent (figure 3).
Its action is such that it causes a capillary rise of 15 cm at 18°C in a 0.1 mm-diameter tube.
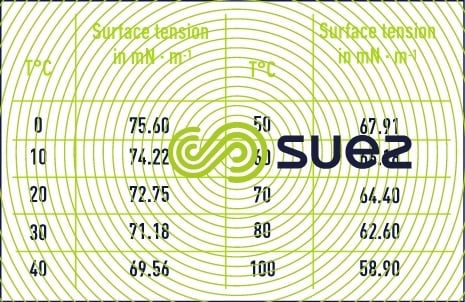
Surface tension decreases when the temperature increases (table 4).
Adding dissolved salts generally increases the surface tension (γ = 74.6 mN.m-1 for an aqueous solution of NaCℓ at 1 mol · L-1, at a temperature of 18 °C).
Other substances decrease it, and these are called “surfactants”(detergents, for example).




electrical properties
dielectric constant
Water’s dielectric constant, which is approximately 80 Farads Steradian per metre, is one of the highest known, which is why water has major ionising power.
water’s electrical conductivity
Water conducts only slightly. The conductivity of the purest water ever obtained is 4.2 micro siemens per metre at 20°C (for a resistivity of 23.8 mega-ohm centimetre). It increases when salts are dissolved in the water (see characteristics solution constants) and it varies depending on the temperature.
opticalproperties
Water’s transparency depends on the wavelength of the light that passes through it. While ultraviolet passes through it well, infrared, which is so useful from a physical and biological perspective, hardly penetrates it. Water absorbs the red and orange parts of the visible spectrum to a high extent, which is the reason for the blue colour of the light that has passed through a thick layer of it.
This transparency is often used to assess certain forms of pollution and, consequently, the effectiveness of purification treatments, and likewise it influences the use of ultraviolet during disinfection.
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












