h2s removal
Reading time:Three types of processes can be considered.
physical removal by stripping
Open air aeration (cascade, spray) can only be considered for low concentrations of H2S because:
- there will not be a 100% removal (except in the event of a very acidic pH);
- discharging H2S into the atmosphere poses environmental problems (odours, toxicity) and requires the stripping gas to be scrubbed using bleach or ozone (see section odour control in water treatment).
Forced aeration in an aerated reactor of the type used to eliminate CO2 would be more efficient but would pose the same problems for the neighbouring vicinity and frequently require the reinstatement of the treated water’s carbonate balance; accordingly, it is not often used.
chemical processes
oxidation
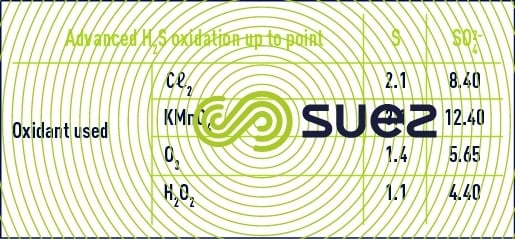
Chemical oxidising reagents are effective but difficult to control: depending on treatment conditions (pH, ORP, etc.), the component used for oxidation can be either elementary sulphur (colloidal), the sulphate ion or intermediate compounds. Table 10 provides the theoretical stoichiometric proportions. In practice, several oxidation compounds will be obtained and the water’s oxidant demand will vary between these two extremes. It is obvious that the cost of this type of treatment quickly becomes prohibitive when the initial H2S content is high.


precipitation using iron salts
The use of ferrous sulphate or ferric chloride, produces iron sulphide precipitates (FeS, FeS2, Fe3S4) which are in themselves colloidal and that, therefore, have to be coagulated, flocculated and then separated. This process is occasionally used in the field of effluents but has not been sufficiently well mastered for use in drinking water treatment.
biological processes
In a slightly aerated medium, sulphur bacteria such as Beggiatoa or Thiothrix can catalyse, using the enzymatic method, H2S oxidation into elementary sulphur (see section sulphur cycle ). Reaction rates and filter seeding will occur as rapidly as in biological iron removal (see Iron removal) ; therefore, the treatment design will be identical (figure 29): in-line aeration and filtration; in this case, the velocity will range from 10 to 20 m · h–1.
When iron is present at the same time, it can then be removed biologically through the same filtering media. When water simultaneously contains H2S, Fe2+, Mn2+ and NH4+, two elementary treatment stages may be considered in series :
- biological removal of H2S, and then of Fe2+, during a first filtration stage after controlled aeration:
- biological removal of NH4+, and then of Mn2+, during a second filtration stage after intensive aeration.
conclusion
The biological method is by far the most cost-effective strategy but has yet to be fully explored and trials will be required in all cases in order to identify operating parameters and permissible filtration rates.
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












