disinfection
Reading time:definition
Disinfection is the final stage in drinking water treatment before its distribution. Disinfection is used to remove pathogenic micro-organisms from the water. However, it should be noted that disinfection is not the same as sterilisation (sterilisation = destruction of all germs present in a medium) and therefore a few common germs may remain in the water following disinfection (see the section Oxidation and reduction and the chapter Oxidation-disinfection).
bactericidal effect – remanent effect
The disinfection of water comprises two important steps that refer to two different properties of a given disinfectant:
- bactericidal effect : this is the disinfectant’s capacity for destroying microorganisms during a specific stage of the treatment;
- remanent effect : this is the disinfectant’s capacity to persist in the water in the mains distribution network and its ability to maintain the water’s biological quality at the consumer’s tap. Disinfection provides both bacteriostatic protection against bacterial regrowth as well as a bactericidal effect against low level and occasional pollution affecting the mains network; at the same time, disinfection blocks the development of micro-invertebrates which could have passed through the plant in resistant (endospores) or reproductive forms (cysts).
Table 6 recaps the qualities of the principle disinfectants used (see Oxidation and reduction).



general conditions required for satisfactory disinfection
In order to be effective, disinfection must be carried out on good quality water. The suspended solids content must be kept as low as possible and equal to no more than 1 mg·L–1. In effect, bacteria and especially viruses collect on suspended solids which can protect them from the effect of disinfectants.
OM, TOC and especially AOC or BDOC levels must also be as low as possible or the water will have a higher disinfectant demand, resulting in:
- the need to overdose of this reagent;
- difficulty to maintain a residual level through the mains without topping up at various points throughout the mains;
- bacterial to regrowth as the water is distributed;
- production of harmful disinfection by-products.
However, endeavours to reduce the formation of THM must not jeopardise the effectiveness of the disinfection itself.
conditions governing the application of the various disinfectants
As discussed in section basic concepts on disinfection, satisfactory disinfection via oxidising reagents requires a residual concentration C combined with a contact time T; resulting in the C·T factor:

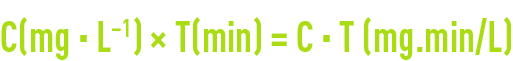
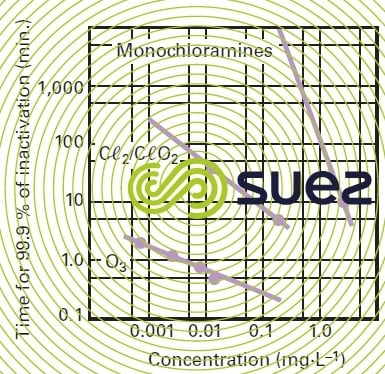
This value will vary depending on the target micro-organism, the type of disinfection used and the temperature. For instance, figure 11 details the conditions applicable to the elimination of 99.9 % (3 log) of an Escherichia coli population; more generally, sections oxidation and disinfection using chlorine, oxidation and disinfection using chlorine dioxide, oxidation and disinfection using ozone and ultraviolet disinfection, detail the conditions applicable to the use and performance of the various disinfectants including UV irradiation, depending on their conditions of use (CT, temperature, pH, dosage) and on the micro-organisms targeted.


For guidance, the following rules apply:
chlorine
By maintaining a 0.5 mg · L–1 residual of free chlorine for a 30-minute contact time (C·T = 15) and a pH of less than 8, pathogenic bacteria and poliomyelitis viruses will be eliminated; however, there is a risk of generating taste and THM in the mains system in the presence of TOC.
chlorine dioxide
An effective level of protection is provided by maintaining a rate of 0.2 mg/l for 15 minutes (C·T = 3). The residual effect is significant. However, it is not recommended and the use of a too high amount of CℓO2 for disinfection purposes may even be banned in some countries like France. The CℓO2 oxidising action on OM releases the CℓO2– ion that has been recognised as toxic and that imparts an unpleasant metallic taste to water.
ozone
For the purpose of eliminating pathogenic bacteria and polioviruses, maintaining a 0. 4 mg · L–1 residual for 4 minutes (C·T = 1.6) is recommended. At 5°C, a C·T equal to 2 will be required in order to ensure that Giardia cysts are eliminated; this value must be higher than 15 in the case of Cryptosporidium oocysts. Under these conditions, it is essential to ensure that the use of this type of treatment does not create unwanted oxidation by-products, particularly bromates (BrO3–) that are regarded as dangerous at levels lower than < 10 µg · L–1. In effect, this type of observation rise to the "multiple barrier" treatment concept already mentioned: chemical disinfection against bacteria and viruses by applying customary criteria, whereas cyst removal efficiency will mainly rely on filtration effectiveness (through fine granular material or, better still, through clarification membranes) or even UV irradiation.
For ozone disinfection, the water must be free of soluble manganese (Mn2+) prior to ozonation. If this is not the case, the water will take on a pink tint. This colour will then develop into a light-dark brown as the MnO2 precipitates.
In view of the comments set out in the preceding paragraphs, it is not advisable to use ozone as the final stage disinfectant. The water then has to be filtered through granular activated carbon with the aim of reducing BDOC levels in order to limit the risk of regrowth in the distribution mains, followed by a ‘belt-and-braces’ chlorination.
chloramines
Chloramines are virtually no longer used for their bactericidal effect (far too weak) but more as a “bacteriostatic” measure in the distribution network because of their strongly persistant residual effect, especially when distributing relatively hot water (25°C or higher) because chloramines are more stable than free chlorine at these temperatures. In countries where a high level of residual disinfectant is acceptable at the consumer tap, a greater use is being made of chloramines after disinfection using ozone or chlorine (bactericidal effect).
UVradiation
UV disinfection has been described in the section ultraviolet disinfection along with the recommended target dosage based on the treated water transmittance, target microorganisms and the elimination performance sought.
For drinking water disinfection, in view of the excellent transmittance levels (tr > 90 % m–1) in water < 1 NTU, a dosage range of 20 to 40 mW·s·cm–2 can be recommended and the use of medium pressure lamps will become obligatory (small number of lamps – small overall dimensions …), as summarised in table 7:



Conditions of Use :
- Reactor geometry and, therefore, hydraulics, are important: nowadays, contact chamber effectiveness can be evaluated using computational fluid dynamics (CFD, see sizing principles) that take the system’s hydraulics into consideration (and, therefore, the time taken by the water to flow through the UV lamps’ zone of influence), the beam’s power and its attenuation after it has been absorbed by the water and its dissolved substances.
- Good iron and colour removal are required in order to achieve satisfactory transmittance and also to avoid severe fouling of the quartz sleeves protecting the lamps.
- In view of the large amounts of heat given off, care needs to be taken to ensure that the water is slightly aggressive in order to avoid rapid sleeves scaling.
- In any case, reactors must be equipped with an automatic quartz sleeves cleaning system.
Advantages and drawbacks :
The only disinfectant that does not create damaging by-products and that effectively inactivates all micro-organisms including protozoan cysts, UV irradiation has two major drawbacks if due care is not taken :
- It is not possible to check the effectiveness of the delivered dose by measuring the residual as in the case of chemical oxidants; therefore, it is essential for the reactor to be equipped with UV intensity sensors (if possible, 1 for each lamp) to ensure that the true radiation emitted by each lamp can be continuously checked so that:
- normal lamp ageing can be monitored (compensated by increasing the current applied to the lamp);
- any lamp failure will be instantly indicated, so that the standby reactor can automatically be brought into service or the faulty lamp replaced (only a few minutes shutdown required);
- no residual effect and, except in the case of short and particularly well maintained distribution network, UV disinfection must be combined with another disinfectant that has a residual effect (Cℓ2 – CℓO2 – chloramine). Therefore, the UV reactor must be installed after polishing but before injecting this last reagent.
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later














