problems linked to water quality and operating conditions
Reading time:Scaling and corrosion cause a surface state degradation and thus an increased risk of biofilm formation.
scaling
Scaling is a phenomenon which corresponds to the precipitation of mineral salts according to their solubility and their concentration.
Scaling results in:
- a degradation in the state of surfaces;
- losses in thermal exchange;
- risk of corrosion under the deposits formed;
- increased cooling towers maintenance needs.
Scaling occurs when:
- the temperature rises;
- salt concentration increases;
- pH varies;
- CO2 content falls.
The phenomenon is based on the formation of insoluble salt deposits on the surface of materials, most often via the modification of the calco-carbonic balance:


It can also result from the solubility limit of a salt being exceeded: as soon as a sufficient quantity of ions is reached in water, a precipitate can form. In the case of calcium sulphate for example, the product of ion concentration is greater than the solubility constant, meaning that precipitation of the excess salts will occur.


Generally speaking, the types of scaling encountered are listed below:
- calcium carbonate: low product of solubility / hard and adhering deposits;
- calcium sulphate: precipitation at high temperature / hard and adhering deposits;
- silicate scaling: depending on pH and temperature, calcium or magnesium silicates can form, resulting in scaling which is difficult to eliminate;
- calcium phosphates: in the presence of hard salts, phosphates form sludge which is only slightly soluble;
- magnesium hydroxide (OH)2 : non-adhering deposits.



corrosion
The corrosion phenomenon is reflected by a physicochemical interaction between a metal and its surrounding environment which results in an electron transfer from an anode to a cathode.
Corrosion results in:
- a degradation in the state of surfaces;
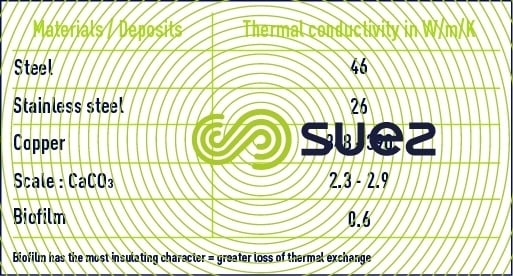
- losses in thermal exchange;
- the destruction of the metal (M) via the release of metallic ions into the water;
- an increase in cooling tower maintenance needs.
The corrosion process can be summarised by the reactions below:

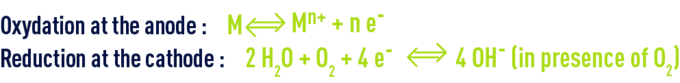
This results in material destruction and / or the formation of corrosion products from the metal.
The main causes of corrosion are the diversity of materials of construction, oxygen content in water or strong oxidizing compounds, and the concentration in strong acid salts (Chlorides, Sulphates, Nitrates). Consequently, corrosion will depend on:
- the metals or alloys used (metal work; surface conditions; metals present);
- operating conditions (flow rate, surface conditions, particularities of the circuit, etc.);
- water quality:
- oxygen content;
- water salinity;
- temperature;
- pH;
- …
Several forms of corrosion exist:
- uniform or generalised corrosion: uniform loss of thickness;
- localised corrosion;
- galvanic corrosion due to the presence of different types of materials;
- crevice corrosion (due to deposits);
- pitting corrosion.
Physical / mechanical causes also exist:
- erosion/abrasion/cavitation corrosion: friction and wear and tear;
- stress corrosion: cracking.
Due to their metabolic activities, certain bacteria can provoke corrosion phenomena:
- iron bacteria : Fe2+ à Fe3+ ;
- sulphate-reducing bacteria : MgSO4 à H2S;
- bacteria known as “sulphur bacteria” : H2S à S or H2SO4;
- nitrobacteria : NH4+ à NO3-;





bio-fouling: bio-film and Legionella
Micro-organisms are involved in environmental cleaning phenomena. Depending on its origin, water will contain more or less micro-organisms. They will have the same type of activity in cooling towers as in their original environment.
The micro-organisms which are of interest to us are those which are at the origin of the problems considered, or those which are indicators of an unfavourable evolution of the environment concerned. They consist of:
- bacteria;
- moulds;
- algae;
- protozoans.
In general, bacterial growth can result in:
- bio-film formation Þ clogging, disruption of manufacturing processes, poor quality;
- material corrosion Þ piercing;
- the degradation of raw materials Þ disruption of manufacturing processes, organoleptic problem;
- the contraction of illnesses infection, Legionnaire’s disease.
Micro-organisms are characterised by the fact that they multiply more or less quickly depending on the conditions encountered in circuits. Among them, the micro-organisms encountered most frequently are bacteria. The presence of micro-organisms in cooling towers can result in the formation of a highly insulated deposit, typically known as a bio-film.
Bacterial growth will depend on several parameters which, if they all reach an optimum value, will permit maximum growth. These parameters are:
- temperature;
- pH;
- salinity;
- redox potential (level of oxygenation of water);
- the presence or absence of inhibitors (biocides) or growth factors (vitamin “equivalent”);
- organic nutrients: the simpler the molecule, the easier it is to be assimilated by a large number of species;
- symbiosis or competition between microbial species.








Certain bacteria can cause body functions to fail, even resulting in death. Since1998, air cooling towers are considered capable of being at the origin of pathology given that water from the tower can contain pathogenic organisms, in particular Legionella. If all of the conditions are present, these bacteria can provoke an illness: Legionnaire’s Disease.
LEGIONELLA RISK: the sites with the highest risk are those where systems could be contaminated alongside a sensitive population. We are all exposed to Legionella, but we don’t all catch Legionnaire’s Disease! For this to occur, all of the factors must be present. Let your doctor know of your exposure to the danger and the risk incurred as a preventive measure.
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












