pollution generated by water treatment
Reading time:When a reagent is added to water, it can create two forms of pollution: impurities contained in the reagent itself and the products generated by the reagent’s reaction with the organic matter contained in the water.
impurities contained in the reagent
In many countries, the use of a reagent will be subject to health authority approval. For each reagent, legislation can impose a maximum impurity level that has to be met by the producer. Products must undergo accurate analysis. When impurities are found, it is essential that checks be carried out to ensure that the intended treatment system can eliminate these impurities.
mineral coagulants
Some coagulants are prepared using ore or metals that are capable of containing impurities in quantities that cannot be ignored: bauxite acid process used to prepare aluminium sulphate, scrap metal used to prepare ferric chloride. This process will also dissolve impurities (tungsten, manganese, arsenic).
Other coagulants are prepared from by-products produced by another industry. Chlorinated copper as is prepared from iron sulphate produced by the titanium industry and can contain relatively high levels of manganese.
polyelectrolytes, additives
Synthetic polyelectrolytes are prepared by polymerising monomers (e.g. acrylamides, amines…).
When processing stormwater, each country’s legislation can impose the type of monomer to be used, the acceptable residual monomer concentration in the polymer and/or maximum treatment rates applicable (in France, this is the case of polyacriylamides: 0.025% of acrylamide monomer and 0.4 mg·L–1 as the maximum amount that can be used in order to satisfy the European parametric value of 0.1 g·L–1 in distributed water).
chlorine ant its derivatives
Depending on its origins, liquid chlorine in particular, sometimes contains various impurities such as: Br2, CCℓ4, CHCℓ3, HCℓ and hexachloroethane, hexachlorobenzene, iron salts.
Similarly, CℓO2, depending on how it is prepared, can contain more or less chlorite and chlorate and, as it reacts with the OM in water, it will "revert" to chlorite. Sodium hypochlorite (bleach) also contains chlorates.
lime pH correction
Quick or slaked lime is rarely more than 93% pure and its impurities (mainly insoluble CaCO3, SiO2…) can significantly contribute to turbidity in drinking water when using lime water that has not been clarified in a saturator.
oxidation by-products
Section Oxidation and reduction, describes the reaction mechanisms that occur between the various oxidants-disinfectants used and pollutants found in water.
As emphasised in organic impurities, the injection of an oxidant (Cℓ2, H2O2, O3) as a disinfection agent, because it mainly reacts with organic matter in water, can create oxidation by-products (e.g. THMHAA, HAN…) some of which are dangerous to human health (carcinogenic and/or mutagenic effects) while others constitute sources of unpleasantness (taste …). This is mainly the case of chlorine and hypochlorite. However, no oxidant is free of by-products; for instance, ozone in some pH or temperature ranges (see oxidation and disinfection using ozone), is capable of oxidising Br- into a bromate (PV = 10 µg·L–1).
The reaction leading to the formation of THM can take place direct through the action of the chlorine present in water as CℓO–, or via another halogen (bromium or iodine) that may have been displaced by chlorine as XO–, depending on the basic reaction:

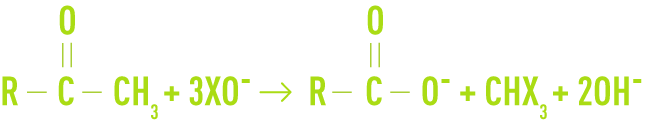
Organic matter containing carbon and giving rise to this reaction mainly comprises methylketones or, more generally, any organic product that, through oxidation, can be converted by oxides to produce methylketones. These THM precursors are mainly found in humic substances and in OM derived from algae.
When organic matter is chlorinated, this also results in the formation of other compounds that have not been identified to date (figure 10). These oxidation by-products are now systematically looked for and their levels are covered by standards that are variable and changing depending on the country concerned.

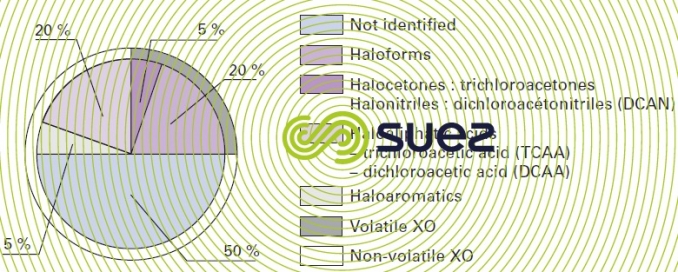

Modern treatment systems (see chapter Drinking water treatment) have been designed with a view to limiting these by-products as much as possible. However, as emphasised by the WHO, this research must not be undertaken to the detriment of proper disinfection (e.g. Cholera in Peru in 1991).
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












