nitrogen cycle
Reading time:Figure 4 is a schematic diagram of the nitrogen cycle.

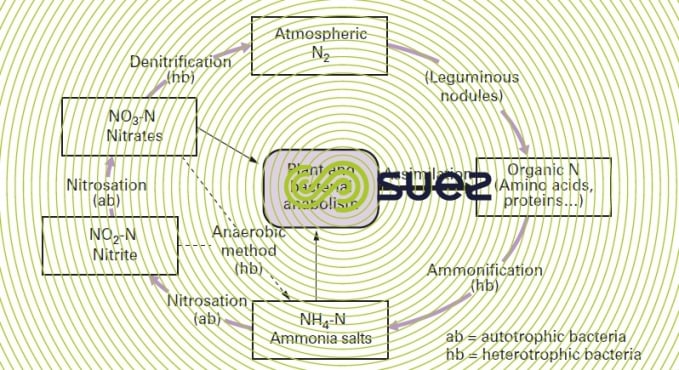

In an aerobic medium, organic nitrogen matter converts into ammonia salts (ammonification) which in turn convert into nitrites and then into nitrates by consuming oxygen. Nitrification encompasses these two reactions:
- nitritation is caused by the action of nitrous bacteria: Nitrosomonas, Nitrosocystis, Nitrosospira, Nitrosoglea…;
- nitratation is caused by the action of nitric bacteria: Nitrobacter, Nitrocystis, Bactoderma, Macroderma…
All these bacteria are autotrophic and strictly aerobic. These bacteria use the energy produced when ammonia and nitrites are oxidised to reduce the mineral carbon released by either carbon dioxide or by carbonates.
In order to achieve a complete reaction, 4.6 mg of oxygen are required for every mg of nitrogen to be oxidised according to the following simplified reaction :


In reality, oxidation of all the nitrogen ammonia is not continued up to the nitrate stage (formation of intermediate organic compounds that make up the bacterial mass) and, in practice, only 4.2 mg of oxygen are required for every mg of nitrogen to be oxidised.
Nitrification tends to deplete the oxygen contained in waterways in the same way as organic pollution assimilation.
On the other hand, these nitrates constitute a stock of oxygen that can be released through denitrification when reduction conditions re-occur (anoxic). These are not the conditions that one could hope for in rivers; however, these conditions prevail in sediments and these reactions play an important part when waterways recharge aquifers.
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












