process water
Reading time:Cooling water and boiler water requirements and constraints are the same for all types of industry. As seen above, they can be the subject of "interprofessional" recommendations or, conversely, process water will be specific to each type of industry and will then be examined accordingly.
Furthermore, section Industrial effluent of the same chapter addresses effluents generated by the same industries. A degree of cross-checking applies (the quantity of water required, description of the processes that use water and that are, in the main, the same as those that produce effluent…).
brewery and carbonated beverage water
breweries
utilisations
- beer preparation;
- vat, equipment and floor washing;
- cooling;
- if applicable, bottle cleaning.
make-up water quality
Make-up water is covered by professional recommendations that are very closely linked to the quality of the beer produced. Thus, bicarbonates that would precipitate when calcium phosphate rich malt is injected must be avoided in the brewing trade.
As a first approach, the following rules apply:
- systematic removal of bicarbonates (precipitation or acidification and degassing);
- the lowest possible Mg concentration (< 10 mg·L–1);
- SO4/Cℓ ratio better than 1 (smooth beers);
- Na concentration < 100 mg·L–1 in order to minimise bitterness;
- NO3< 50 mg·L–1 et NO2< 1 mg·L–1 (limits applicable to potability and to fermentation toxicity).
quantities used
3 to 5 hL per hL of beer of which:
- beer preparation: 1.5;
- washing: 1.5 to 3;
- cooling: 1 to 2.
carbonated beverages
1 to 5 L of water are required to produce each litre of beverage; a proportion of this water forms part of the finished product and, therefore, this water must meet drinking water standards.
Industrials continue to optimise the quantity of water used; some are close to a ratio of 1L of water per 1L of finished product.
Manufacturers themsleves often establish specific water qualities. These qualities will include maintaining a M-alk. of below 5°F and a total salinity of less than 500 mg·L–1. For cleaning water applications, however, several mg·L–1 of excess residual chlorine may be demanded.
mineral water and spring water
Bottled natural mineral water, spring water and table water are subject to specific legislation that, in rare cases, is less stringent than that applied to drinking water.
Additionally, it is advisable to remove any unstable element such as iron or manganese that could precipitate in the bottles whilst in storage.
dairy industries
Water is used to:
- disinfect equipment and vats;
- clean floors;
- wash products;
- reconstitute milk;
- and for boilers and cooling;
1 to 5 L of water are used by various requirements in order to produce one litre of milk.
sugar mills and refineries
sugar beet mills
Industrial water treatment techniques apply to the purification of the make-up water and of the juice itself. The illustration in figure 18 constitutes a simplified diagram of the various circuits found in a sugar beet refinery. Major water consumers, sugar mills have gradually reduced this consumption through numerous recycling and internal re-use systems:
- beet washing: recycling polluted water via scraped sedimentation tanks;
- sugar beet chip diffusion (preparing the raw juice): processing condensed water containing ammonia for recyling back to the diffusion inlet;
- processing thin juice:
- decalcification of second carbonation juices (protecting evaporation apparatus against scaling);
- juice demineralisation (reducing the amount of molasses);
- removing colour from syrups over activated carbon or adsorbant resins;
- juice concentration, sugar crystallisation accompanied by juice conditioning in the evaporators;
- by-product processing (dregs or molasses) with the aim of reducing the sugar-molasses (sugar loss) rate:
- dregs processed over a cationic resin that is regenerated using magnesium chloride (Quentin process). Replacing Na and K ions with Mg reduces the sugar retained in the molasses;
- ion exchange used to demineralise the dregs or molasses with supplementary treatment if required in order to obtain liquid sugar;
- make-up water:
- for topping up boilers at the start of the sugar processing season or in the event of an accidental lack of return condensates;
- permanent make-up supply to turbo-alternator coolers and beet intake.
In the knowledge that sugar beet contains approximately 77 % water, the sugar industry has a cycle that produces excess water as shown in figure 18.



It should be noted that the excess 28 m³ include 10 m³ that are recycled for sugar beet washing, 8 m³ used for various washing requirements and as boiler make-up water and only 10 m³ returned to the natural environment.
cane sugar houses
Raw juice purification requires similar clarification methods to those used for water:
- lime used to achieve rapid sedimentation of "clarified" juices;
- purified juice flotation in order to separate out "stray bagasse";
- circuit disinfection (essential).
sugar refineries (beet or cane sugar)
Syrup provided by melting sugar and liquid sugars must undergor more or less comprehensive decoloration treatment.
fruit and vegetable canneries
On average, 5 to 40 m³ of water is required for each tonne of canned produce used for cleaning, peeling and blanching vegetables.
At least partial process water softening is frequently required. However, total salinity reduction can be advantageous in order to prevent can corrosion.
textile industries
Water is used for boilers (sometimes requiring large amounts of make-up water), process (dyeing, rinsing) and air conditioning (humidification and dust removal).
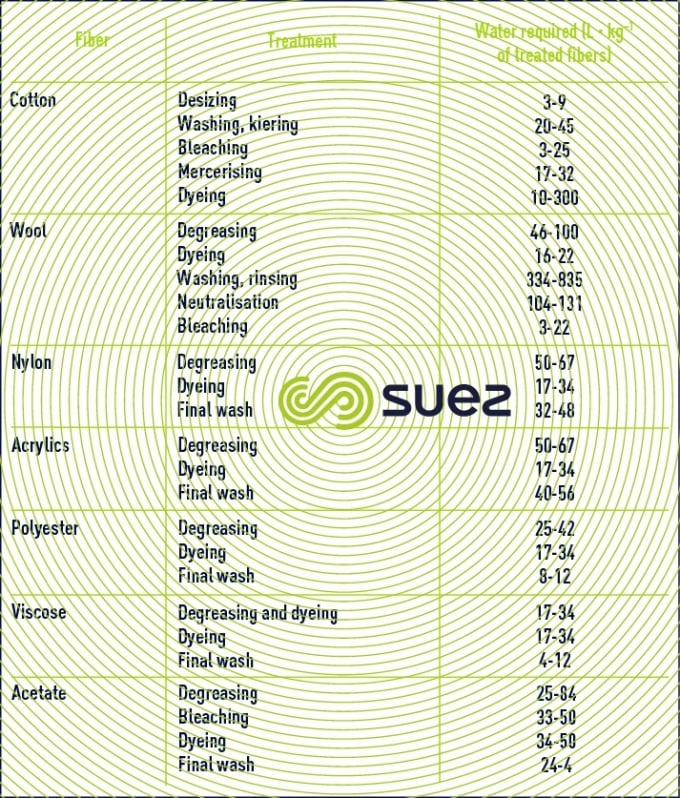
These industries consume major amounts of water; table 22 shows the significant differences found depending on the fibres processed using the following treatments:
- softened or demineralised water used to prepare yarn, especially in the case of synthetic textiles;
- softened water that is often decarbonated and intended for use in fibre bleaching and dyeing;
- demineralised water used in air conditioning systems for spinning and weaving rooms (reverse osmosis, ion exchange).


paper pulp and paper mills
These industries consume huge amounts of water for the following requirements:
- steam production;
- pulp preparation;
- paper manufacture.
make-up water consumption
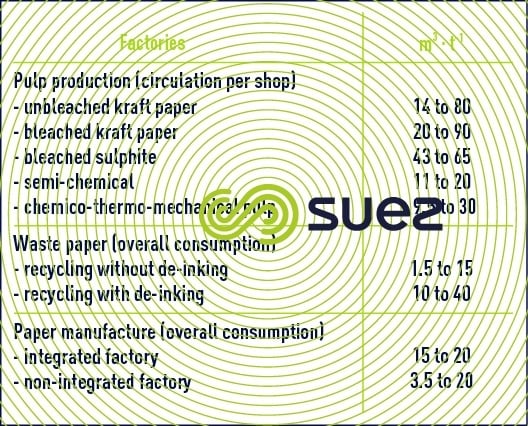
In about 1975, between 100 and 300 m3·t–1 of pulp was required. Since then, thanks to a stringent discharge control policy and, therefore, to intense recycling, these amounts of water have been considerably reduced (see table 23), circulation specific to each shop remaining high.


water quality
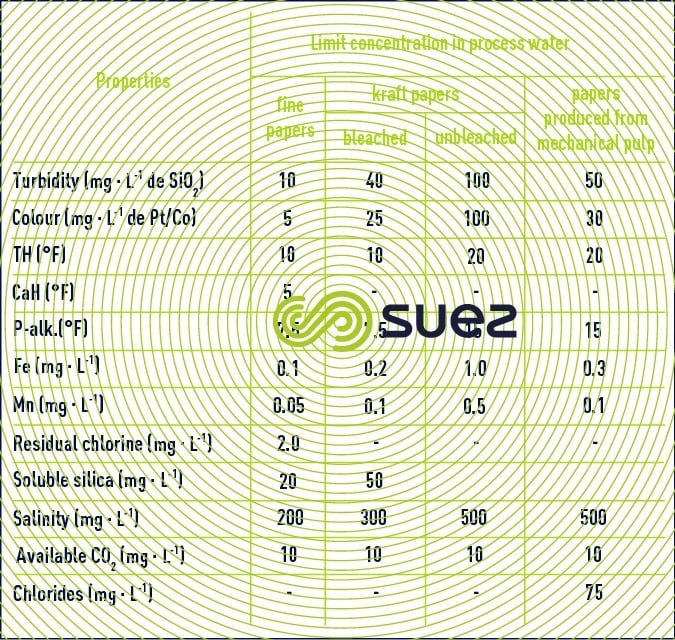
Paper mills mainly endeavour to eliminate turbidity, colour and temporary hardness from raw water.
American recommendations (National Council of the Paper Industry) also recommend some properties depending on paper types (table 24).


oil industry
water or vapor used for injection into oil formations (stimulated oil recovery)
In order to maintain pressure in the formation from which crude is extracted or in the case of highly viscous crudes for the purpose of facilitating their recovery by fluidising viscous crudes, the conventional solution consists in injecting fields that are being worked with either water (freshwater, offshore seawater, well water produced with recycled crude) or with vapor (injecting heat). The outlet is a mixture of crude oil and produced water which, following treatment, can be recycled as injection water.
Depending on the fields concerned, up to 2 or 5 times the flow of recovered crude is required for injections purposes, and up to 10 times the flow of crude oil recovered from mature fields.
This water must:
- not contribute to physical plugging of the formation in the vicinity of the injection well; depending on the porosity (permeability) of the injection well, oil will need to be removed from the water produced and re-injected (< 10 ppm or even < 2 ppm) together with any particles larger than a set limit (e.g. elimination of particles > 2 μ… or at least more than 98% of particles > 2 μ;
- be "compatible", i.e. they must be unlikely to react when mixed with the formation’s water to create insoluble precipitates. For example, by avoiding the need to inject seawater (high SO42– concentration) into formation water that is rich in Sr2+ or Ba2+ or, in that case, to subject this water to a nanofiltration process in order to remove most of those sulphates;
- not inject micro-organisms (e.g. sulphur reducing bacteria) likely to develop into the formation as this often imposes the use of biocides.
With regard to vapor injection (60-120 bar), this vapor is produced from local freshwater or from recycled formation water, depending on the type of boiler used, requiring at least:
- total demineralisation (via reverse osmosis and ion echange) in the case of classic boilers;
- at least silica removal and total softening (usually < 0.05°F of TH) for very high carry-over boilers (approximately 20% entrainment) frequently used for this application.
In the case of alkaline/surfactant/polymer chemical Enhanced Oil Recovery (ASP EOR), their properties allow the extraction of crude oil to be improved but requires that the quality of injection water is appropriate and which, in particular, has a low divalent ion content, generally has no sulphates and is at least partially softened.
Similarly, when injecting low salinity water (TDS or/and Salinity <5 000 ppm ), an additional removal stage of monovalent ions by reverse osmosis is required, either replacing or in addition to nanofiltration.
Non-standard hydrocarbons are removed after injecting steam for heavy and extra-heavy oils, e.g. mining or quarrying for sand or oil shale, by fracking in the case of compact reserves of oil and gas, and oil and shale gas.
Fracking involves boring a horizontal extraction well in bedrock containing non-standard hydrocarbons. Fracking fluid, comprised of a mix of water, sand (proppant) and other additives (lubricant, biocide), is injected under pressure. Along the horizontal wells, several frackers dig into the bedrock in which hydrocarbons are trapped. The fracking fluid mix and hydrocarbons are pumped out through the same wells and separated. The fracking fluid extracted from the wells - or reflux water - contains many dissolved or suspended elements acquired during the fracking process and which must be treated before being reused as fracking fluid or even discharged.
It is estimated that 10,000 to 25,000 m3 of water per well is required for boring and fracking. However this depends on a well's typology.
Fresh water or brackish water is used as fracking fluid following the removal of suspended mater and disinfection.
Reflux water must be treated following the removal of oils, suspended solids and dissolved compounds.
water used in refinery and petrochemical applications
A refinery requires major amounts of water that are essential to refinery unit operation.
The water used can be surface water, ground water or even, in part, seawater.
The following units require make-up water:
- boilers used to produce vapor that is essential to many units but a proportion of this vapour will frequently be wasted if they are semi-closed (high concentration level );
- cooling circuits, which are the main water consumers, account on average for more than 50% the water consumed in a refinery and even more than 90% if they are open.
- the desalter;
- some cracking and refinery process units;
- various process waters (washing, sanitation…);
- fire-fighting systems.
The qualities required will vary widely and depend on the various units concerned (figure 19).

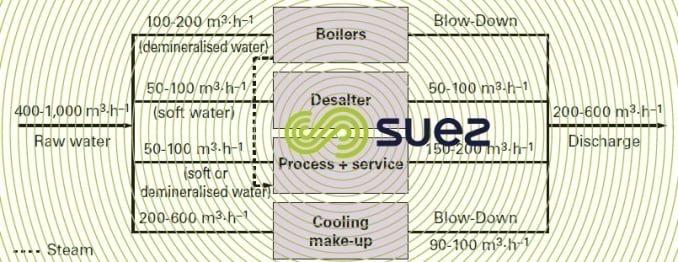

Figure 19 assumes that open recirculating systems are used.
Flows are far higher in open circuits (seawater cooling).
The implementation of recycling within the refinery allows fresh water to be saved and discharge rates to be minimised. Atmospheric topping crude desalters are often fed with re-used water such as acid condensates from cracking units, after stripping out ammonia and H2S. The treated discharge water can feed crude desalters or serve as make-up water for cooling.
steel mills
A major water consumer, this industry has long since constructed open recirculating systems adapted to specific working conditions encountered.
There are two major uses made of water in this industry:
- indirect cooling using exchangers often has to cope with high water temperatures. Make-up water will be decarbonated or demineralised depending on the case. These circuits can also be closed;
- direct cooling, either as gas scrubbing, or product granulation or descaling; during these operations, water becomes soiled and must constantly be treated in the circuit. Make-up water must be either decarbonated or raw water.
coking plant
Indirect primary gas condensation or final direct gas condensation.
Wet dust removal from gas when the preheated coal is fed into the furnace and when coke is removed from the furnace.
blast furnaces
Cooling for blast furnace units such as nozzles, tymps, hot air valves, blast furnace shell.
Blast furnace gas scrubbing.
direct reduction
This process is a very heavy consumer of water:
- washing and cooling iron sponge reduction gases. The amounts of water used (5 to 15 m3·t–1 of sponge) are higher than for blast furnaces and, given the high temperature of these gases, this water is very hot when it is discharged from the washers (50 to 70°C);
- cooling for machinery (compressors and oil coolers).
A significant amount of demineralised water is consumed in the production of vapor used in process gas reforming.
converters
These processes call for major circuits:
- cooling for the hood and the nozzle (sometimes provided by vaporisation);
- gas washing with calorie recovery through partial gas combustion and vaporisation in an LP boiler.
electric furnaces and ladle metallurgy
Fine steels are produced in electric arc or ladle furnaces, heated by induction or arc; this production can be completed by vacuum degassing of the steel. Water is used in three ways:
- normal cooling of furnaces and ladles;
- production of vapor for vacuum forming ejectors;
- cooling molds and electrodes with demineralised water.
continuous casting of slabs and billets
There are three common circuits:
- closed circuit mold cooling using demineralised or softened make-up water;
- machine cooling using an open recirculating system, with appropriate conditioning;
- machine and slabs or billets spraying, creating scaling, slag and flame cutting slurry.
hot mill trains
Two types of circuit need to be fed:
- indirect cooling for furnaces, compressors, motors…;
- direct cooling capable of three functions: metal and rolling stand cooling, steel descaling.
There are many different "hot" mills:
- Strip mills
The largest handle 300 to 1 000 t·h–1 of steel in the form of slabs.
Circulation flows involved:
- direct cooling: 10 to 25 000 m3·h–1;
- indirect cooling: 5to 10 000 m3·h–1.
Motors, oil baths and reheating furnaces have separate cooling circuits.
These cooling circuits are frequently supplemented by:
- automatic scarfing, a process that produces large amounts of granulated slurry;
- slab cooling whether in pools, tunnels or under sprays, uses large amounts of water without creating major pollution.
- Other hot mills
- flat sheet mills or quarto mills;
- blooming-slabbing;
- merchant iron, small beam, section mills;
- rounds or wire mills;
- tube rolling mills.
cold rolling mills
The manufacture of thin rolled products, tin-galvanised or other, requires metals to be pre-treated, degreased and above all, pickled, for instance. The latter operation is increasingly often carried out with hydrochloric acid regenerated in situ.
Demineralised water is required for final rinsing of zinc or tinplating shops. Extremely pure and softened water is also required for preparing soluble oil baths.
copper metalworking
Metal can be worked either dry or wet depending on the type of ore involved. Hydrometallurgy processes that involve sulphuric acid leaching and electrolysis, are used with increasing frequency because they can process poor quality ore and even old floatation residues.
Copper rolling from "wirebars" is used to produce sections, cables or wires.
Roughing operations use hydraulic descaling of the metal surface and result in a water/copper oxide suspension. Copper oxides are products whose recycling can often prove cost-effective. Additionally, operating costs applicable to descaling and cooling circuits are similar to those found in steelmaking.
hydrometallurgy
This industry particularly concerns the extraction of uranium, gold, cobalt.. and makes use of the following elementary processes:
- metal extracted by acid or alkali attack (leaching);
- solid/liquid separation: filtration and/or sedimentation;
- metal concentration; extraction using solvents or ion exchangers;
- miscellaneous precipitations.
The advantage of these processes is that they can be carried out cold and reduce corrosion problems. The technologies used are very often similar to those applied to water treatment and, therefore, can benefit from the experience acquired in our field. The following constitute the most important parameters, especially for liquor clarification:
- suspended solids: after sedimentation has taken place, liquors will still often contain 100 to 200 mg·L–1 and even, occasionally, several g·L–1 of suspended solids. This residual matter causes problems in both direct extraction of the metal and also in purification-concentration using an organic solvent or resins. Many users would like to reduce residual matter levels to below 10 to 20 mg·L–1;
- colloidal silica: silica is present in its ionised form in liquors (silicic acid or fluosilicic acid) at maximum concentrations that can reach 200 to 500 mg·L–1 or in the form of weakly ionised polysilicic acid gel. This colloidal dispersion is electropositive and, consequently, cannot be coagulated using customary techniques. This silica will precipitate over resin or in the presence of solvents;
- calcium sulphate: limestone and dolomitic ores processed with sulphuric acid will produce liquors that are supersaturated with CaSO4, a source of scaling and precipitation. This supersaturation has to be broken down by seeding gypsum micro-crystals before embarking on any other treatment;
- organic matter: organic matter can be deleterious in two cases:
- during liquid-liquid extraction, the residual solvent will adversely affect metal precipitation, especially during electrolysis;
- through fixing on carbon; some organic matter that is adsorbed may not be eluted upon chemical regeneration. Other organic matter will form a non-adsorbable complex with the metal.
the automotive and aircraft industries
The automotive industry includes mechanical factories: construction of engines and gear boxes, bodyshops, assembly shops and many independent subcontract workshops. Their make-up water requirements will vary widely depending on their respective situations.
Nevertheless, we can highlight three groups:
- cooling, mainly for compressors and air conditioners;
- preparation of various electroplasty and paint baths that usually call for demineralised water;
- low mineralisation or softened water for milling and grinding stations.
The aeronautics industry has similar requirements.
electronic industries
It is in this field that the most extreme qualities are required. Most of this water is used for rinsing "silicon wafers" after each elementary component and circuit installation operation.
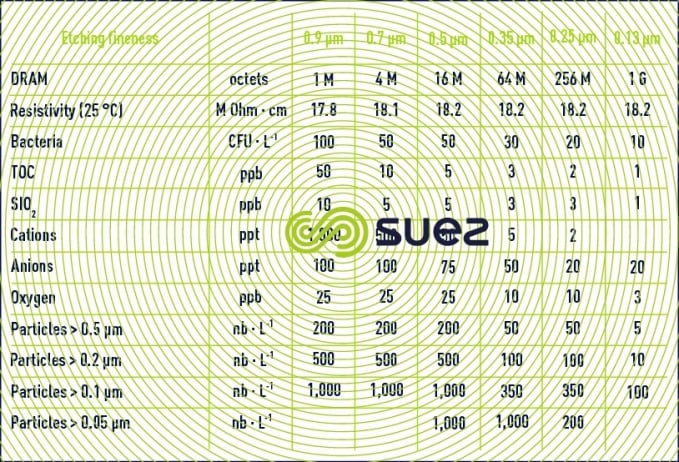
Table 25 illustrates changes to rinsing water specifications quoted in DRAM documentation; the increasingly fine nature of the etching carried out is such that no particulate deposit, bacteria (even dead) or salts capable of creating a significant fault, must be left by this water.
Note: each manufacturer, depending on the type of working document, integrated circuit … he produces, has special requirements but, in all cases, the race towards miniaturisation results in extreme requirements that must be achieved and, in addition, a high degree of reliability is demanded (seeTreatment and conditioning of industrial water). Any quality defect will appear as an increased in the manufactured product rejected and even shutting down production and causing considerable financial losses.


pharmaceutical and biotechnical industries
Both these fields demand water of an extremely high quality:
- water used in the medicines themselves and, especially, those used in injectable solutions; the latter are covered by a range of national or international standards (European , American, Japanese, pharmacopoeia…) see ultra pure water used in semiconductor and pharmaceutical industries and must especially be free of:
- any bacteria ;
- any viruses ;
- these solutions must also be apyrogenic (they must not induce any fever when injected), implying the absence of antibodies or of all or part of membrane or intra-cellular matter from bacteria killed during sterilisation ;
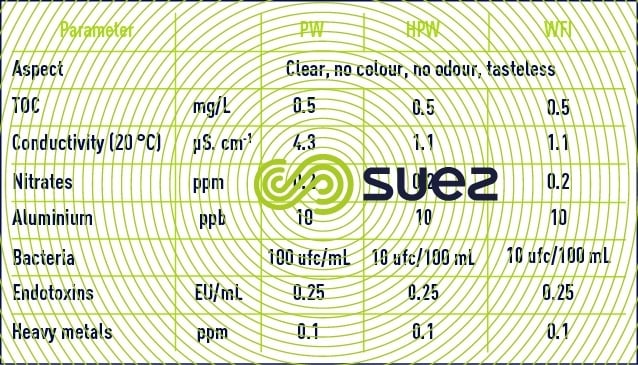
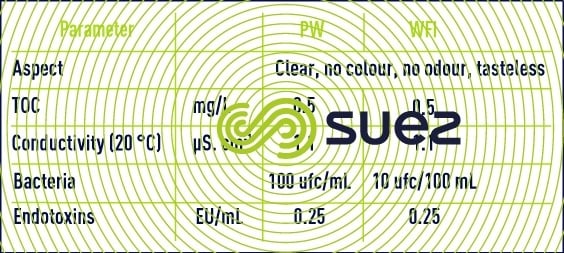
The European Pharmacopoeia defines three water qualities: purified water (PW), highly purified water (HPW) and water for injection purposes (WFI). Their specifications are compared in Table 26.
The American Pharmacopoeia does not make a distinction between highly purified water and purified water. A few recommendations given by USP 34 pharmacopoeia are mentioned in Table 27.
- water used for proliferating certain strains of bacteria or yeasts from which the active principles are then extracted.
In both cases, production lines must be adapted to precise industrial specifications; nevertheless, as in the case of the electronics industry, membrane combinations are essential in order to guarantee quality and reliability (see ultra pure water used in semiconductor and pharmaceutical industries).




Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












