notions of heat
Reading time:Flammability limits: the mixture of a comburent (fuel) and a fuel has a flammability range that runs from a lower limit e Li (below which the mixture is too poor to burn) to an upper limit Ls (beyond which combustion cannot take place because there is not enough air).
Table 86 provides the flammability range limits for the four mixtures of hydrogen or methane with air or oxygen (pure): Li and Ls are expressed as a %


; the table also provides data on methanol/ethanol or NH4 combinations in air.
The ignition temperature represents (even when very localised) the lowest temperature at which a mixture situated between limits Li and Ls will ignite (blow up).



Combustion heat is the amount of heat released during the combustion of a substance, pursued up to its total oxidation. In the case of a compound, this heat will be equal to the sum of its heat of formation and the heat released by the combustion of each of its elements.
calorific value
The calorific value is the combustion heat expressed in relation to the fuel’s unit of mass or volume (KJ·kg–1, or KJ·m–3, or even mth·kg–1). We have:
- the calorific value on pure fuel : Quantity of heat released through the combustion of the unit of mass or volume of a fuel that is free of humidity, mineral matter and incombustible gases.
- the calorific value on dry fuel that applies to the unit of mass or volume of a fuel from which all humidity has been removedé.
- the as-received calorific value : That takes into account all the fuels in the product unit of mass or volume.
When the fuel contains hydrogen or hydrogen compounds, these will be found as water in the products of combustion. Depending on the state of this water in the products of combustion, gas or liquid, we can define the net calorific value or the gross calorific value.
net calorific value (NCV)
The net calorific value does not include the heat generated by the vaporisation of water contained in the products of combustion (therefore, this water is deemed to remain in the form of vapor).
gross calorific value (GCV)
The gross calorific value includes the vaporisation heat of the water formed during the combustion process. The definition of the GCV therefore assumes that all the water from the humidity in the fuel and from combustion can be found in the condensed state in the products of combustion. The water contributed by the comburent (wet air) is supposed to remain as vapour.
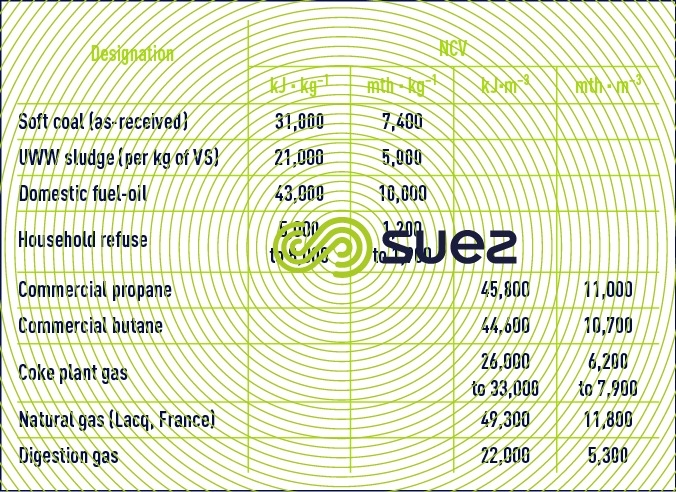
The difference between the NCV and the GCV is the latent water vaporisation heat. The NCV, alone used for plant design, is deduced from the GCV after establishing the hydrogen and water content of the fuel. Table 87 provides a few average values for the NCV.
The difference between the GCV and the NCV based exclusively on dry organic matter is in the region of 10 to 15 %; for domestic fuel oil, it is 5 to 9 %.
Note : economic studies normally use the tonne of oil equivalent (toe) concept. 1 toe is equivalent to the energy value of one tonne of liquefied gas or 1,000 m3 of natural gas, i.e. 36·103 kJ.


combustion
Among conventional forms of combustion, in wich the comburent is the atmospheric air, we have:
theoretical combustion
The quantity of air involved is equal to the combustion capacity of the fuel (see below). This combustion is incomplete,
oxidising and semi-oxidising combustion
The quantity of air involved is greater than the combustion capacity of the fuel. This combustion with excess air is complete in the first case, Incomplete in the second,
reducing and semi-reducing combustion
The amount of air provided to the fuel is below its combustion capacity; it is completely absorbed in the first case and partially in the second.
combined combustion
Mixed combustion produces fumes contained unused oxygen and unburnt residues. This type of combustion is occasionally found in practice on the grounds of technical difficulties.
Neutral combustion is a combustion that takes place completely with the quantity of comburent required and strictly sufficient to achieve neutral combustion. This type of combustion corresponds to a theoretical concept and is only achievable with difficulty in practice. However, it can be used to establish a certain number of parameters that are characteristic of a combustion.
A fuel’s combustion capacity is the amount of air that is strictly necessary to guarantee the neutral combustion of a unit of this fuel.
As an initial approximation, for liquid and solid fuels, we can assume that this combustion capacity is in the region of 1 Nm3 of air per kg for a NCV of 4 000 kJ·kg–1.
For gas fuels, the value remains the same but applies to the m3 under normal conditions and not to the kg:
1 Nm3 of air per Nm3 of gas for a gas having a NCV of 4 000 kJ per kg Nm3.
In urban waste sludge, it will be in the region of 6.5 Nm3·kg–1 of VM.
The fume producing capacity of a fuel is the quantity of fumes resulting from this fuel’s neutral combustion. In practice, we generally use the idea of fume forming capacity on wet fumes. In this case, it is assumed that the water vapor has not been condensed. As an initial approximation, it can be determined using the Véron formula that provides:
- for a solid fuel: 1 Nm3·kg–1 per 3,500 kJ·kg–1 of NCV,
- for a liquid fuel: 1 Nm3·kg–1 per 3,800 kJ·kg–1 of NCV,
- for a gas fuel: 1 Nm3·Nm–3 per 3,500 kJ·Nm–3 of NCV,
(not applicable to lean gases, having a NCV of less than 8,000 kJ·Nm–3).
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












