cathodic reactions and corrosivity in water treatment
Reading time:corrosion in iron in the absence of oxygen
Using the concept of a noble metal for the purpose of defining a metal’s tendency to corrode only applies in pure water at a temperature of 25°C. A complete definition of a metal’s tendency to corrode in water must take all the water’s properties into account. For instance, we can quote the case of iron exposed to a captive mass of pure water in the absence of dissolved oxygen at 25°C.
Under these conditions, iron has the following anodic reaction :


The cathodic reaction is the reduction of hydrogen ions to form hydrogen gas :


Therefore, the global corrosion reaction can be written as follows :

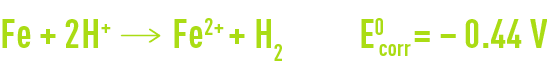
The Nernst equation for this system is written as follows :

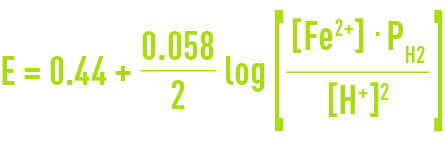
where PH2 is the partial pressure of hydrogen gas, set at 1 for the purpose of this example.
As the reaction takes place, an electric current circulates between two half-cells, depleting H+ and creating an accumulation of Fe2+. The reaction continues until it produces Fe2+ and H+ ion concentrations that bring the potential E to 0.0 volts, at which point, the system is in equilibrium. This occurs more or less under the following conditions, on the assumption that no Fe2+ or H+ is lost:

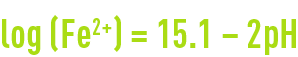
There is no practical stability range common to iron and water below a pH of 10.5 when iron solubility will be equal to 10–6 mol · kg–1, a figure that applies to negligible corrosion.
At a lower pH, ferrous iron remains soluble and there is no formation of any deposit; thus a slow and generalised corrosion persists as the result of the presence of an infinite number of cathodes and anodes that co-exist and occupy the entire surface area of the metal.
corrosion in iron in the presence of dissolved oxygen
electrochemical mechanism
Dissolved oxygen plays a major and complex role in the corrosion mechanism and especially in the case of iron. Oxygen takes part in the half-cell cathodic reaction according to the following reaction :


The half cell cathodic reaction potential will then be established through the Nernst equation :

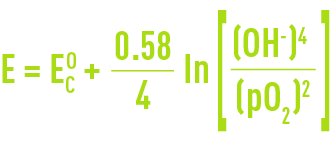
The following factors affect the cathodic potential :
- the OH- ion concentration which is in turn determined by the pH:
- partial oxygen pressure pO2.
When this potential is higher than that of the metal electrode, the corrosion reaction’s net potential will be positive and the reaction will take place. Under normal conditions, the reaction's potential will be + 1.21 volts, a sign that the aerated water reacts in a strongly thermodynamic manner to the iron.
role played by corrosion products
The products generated by half-cell anodic and cathodic reactions will be Fe2+ and OH- ions respectively. The reactions of these species oxygen and other compounds with the water will play a crucial role in the formation of either protective layers or of harmful deposits.
The dissolved oxygen is involved in secondary reactions, forming corrosion products at the anode :

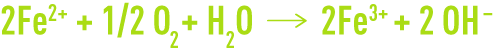
The solubility of species that contain Fe3+ ions is far lower than that of Fe2+ compounds. When deposited, Fe3+ ions will slow corrosion down, thus explaining the essential role played by oxygen in many corrosion inhibitor mechanisms.
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












