general corrosion
Reading time:If the products of the corrosion reaction are soluble and if the composition of the corroded material is homogenous, corrosion will be uniform over the entire surface. This type of attack is known as "generalised corrosion". We give below a few examples.
acid corrosion
Acid corrosion occurs when the H+ concentration in the aqueous medium is sufficiently low to allow the water to convert to hydrogen through a reduction cathodic reaction. Acid corrosion is normally "generalised" when corrosion products are sufficiently soluble in an acid medium for there to be no metal oxide deposits. With acid corrosion, the cathodic reaction produces molecular hydrogen that diffuses through the metal, causing it to become brittle and fracture at high temperature or in the presence of a very strongly acid pH (pickling ….).
alkaline corrosion
Alkaline corrosion is caused by the amphoteric nature of some metals. For instance, aluminium hydroxide precipitates and forms an inhibiting layer through the following reaction :

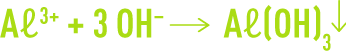
However, with a high pH, aluminium hydroxide can resolubilise through the following reaction :


In strongly alkaline solutions, this resolubilisation causes alkaline corrosion. Aluminium and zinc and iron in boilers are particularly sensitive to this type of corrosion, and even iron in boilers:
- either due to an excessively high pH in circulating water ;
- or to localised overheating and to the simultaneous excess concentration of OH- ions in the water.
corrosion involving complexing agents
Complexing agents are chemical agents that form soluble and stable complexes with metal ions. These agents are used in boilers or other applications to prevent the formation of deposits or to dissolve existing deposits. Most of these react on passive layers and may, therefore, cause or contribute to corrosion of the base metal.
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












