kinetics of the corrosion reactions
Reading time:The difference between corrosion and immunity can be defined by the electrochemical potential of the corrosion reaction (see above). However, the definition of passivation requires a study of electrochemical reaction kinetics and of the concept of polarisation, i.e. the variation in the electrodes potentials caused by a net current. The relation between the half-cell rate of reaction and its potential is given in the general form :


where, for an electrochemical reaction, E is the potential recorded, E is the thermodynamic potential and the rate of the reaction is defined by the stream of electrons (i) between the cathode and the anode.
An Evans diagram can be used to outline the impact polarisation has on the corrosion process, as seen in figure 2.

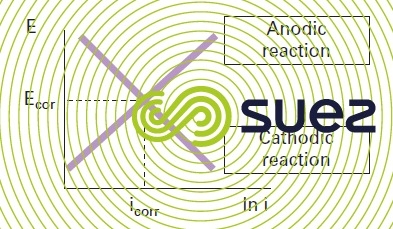

The potential (represented by the ordinates axis) is the potential actually applied whereas the current generated by the anodic and cathodic reactions will run in the opposite direction. The Evans diagram represents the absolute value of the half-cell current. Thus, electro-neutrality requirements are met at the point where the anodic and cathodic reaction lines intersect. Potential (Ecorr) and current (Icorr) at this point define the corrosion potential and the current/rate of the overall electrochemical mechanism.
If the cathodic reaction is polarised, the cathodic curve of the Evans diagram will shift towards lower current values with a constant potential b → c. Figure 3 illustrates the net effect.

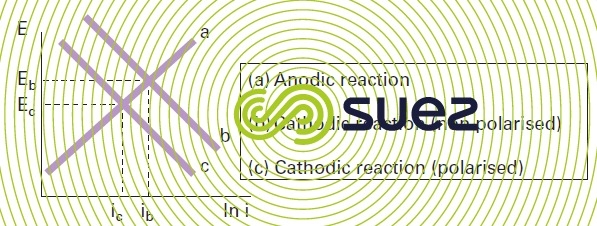

The visible result of cathodic polarisation is such that both the corrosion current (Icorr)) and the corrosion potential (Ecorr) are reduced. Similarly, if a system subject to corrosion undergoes anodic polarisation, the anodic curve of the Evans diagram will shift towards the lower figures a b leading to a corrosion current reduction but to a corrosion potential increase (figure 4).

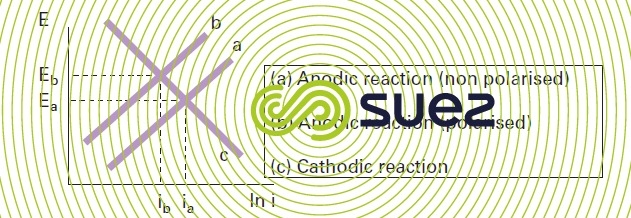

These constitute the fundamental principles of the experimental classification of "anodic" or "cathodic" corrosion inhibition processes according to the type of polarisation observed.
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












