copper alloys
Reading time:brass
Brass consists of copper-zinc alloys and the following compositions can be used (table 4) :

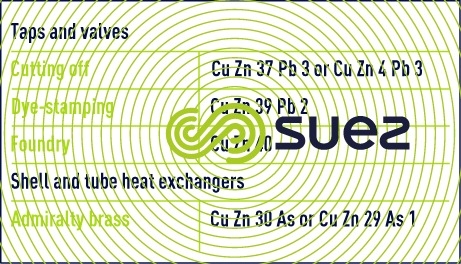

The dezincification phenomenon, or zinc dissolution releasing residual Cu, will occasionally occur in high salinity, low hardness water. It renders metal porous and fragile. There are brasses that are not dezincifiable (Cu Zn 35 Pb 2 As) that can be used to avoid this accident.
In the presence of high chloride water or seawater, exchanger tubes must be constructed of admiralty brass or titanium.
cupro-nickel alloys
Good cupro-nickel alloy behaviour can be affected by the presence of traces of ammonia (> a few mg·L–1) and traces of H2S in seawater.
Beyond a few mg·L–1 of NH4+, crevice corrosion may occur but can be eliminated by adding traces of Fe2+.
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












