nitrogen conversion
Reading time:In natural water, nitrogen is usually present in its ammonia and/or nitric form; there is no need, therefore, to take the ammonification and assimilation phases quoted in respect or UWW and IWW (see suspended growth (activated sludge) process) into account and the only treatments applied to storm water are those that concern the elimination of NH4+ and NO3- ions.
nitrification
theory of the process
Ammonium can be eliminated:
- either by the physical-chemical method: break-point chlorination (see the oxidants and disinfectants); this is a costly treatment and it creates organochlorine products (especially THM); therefore, it is used less and less, except on extremely clean water that contains little NH4;
- or by the biological method: nitrification, that consists in using enzymatic oxidation to convert ammonium into nitrites and then into nitrates under the effect of specific bacteria.
Nitrifying bacteria are autotrophic; in this precise case, in order to form their substance from the mineral carbon contained in water (CO2, HCO3-), they take the energy they require from the reduced forms of nitrogen:
- ammonium converted into nitrites ("nitritation") under the effect of bacteria of which Nitrosomonas is the predominant type:

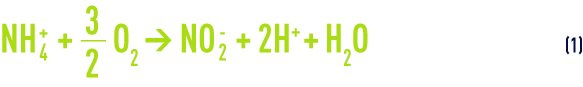
- Followed by the conversion of nitrites into nitrates ("nitratation") by other bacteria of which the predominant type is Nitrobacter:


The overall nitrification reaction (see also suspended growth (activated sludge) process) is obtained by adding, term by term, equations (1) and (2):

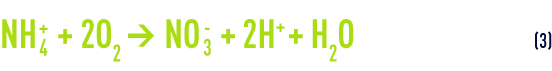
In storm water, ammonium content is usually expressed in NH4+ ions according to standards (whereas in UWW and IWW, the unit used tends to be mg · L–1 of nitrogen ammonia). Reaction (3) leads us to conclude that we need approximately 3.6 g of oxygen to oxidise 1 g of NH4+ ions (or 4.6 g for 1 g of N-NH4) and that this is an acidifying process (releasing H+ ions).
Nitrifying bacteria being autotrophic and aerobic, their utilisation only calls for an O2 input (aeration) plus traces of phosphorus (0.1 to 0.2 g · m–3, as PO43–) in order to be able to synthesise their DNA and their ATP. If necessary, an alkaline product may have to be added to water containing high levels of HN4+ aand low M-alk. in order to maintain pH above 7.2.
nitrification kinectics
As in all chemical or biochemical reactions, a certain time is required before nitrification reactions are completed, unlike the process applicable to biological iron and manganese removal, in this case, the reaction kinetics take place fairly slowly; these kinetics are also strongly affected by the pH and by temperature.
In the optimum pH zone (7.2 to 8.5), we can use an empirical formula to quickly calculate the characteristics of a nitrification reactor; this calculation is based on the following equation

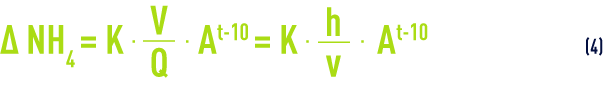
where: Δ NH4 = quantity of ammonium ions to be eliminated (mg · L–1)
Q = water flow rate (m3 · h–1)
V = contact mass volume (m³)
h = contact mass height (m)
v = water speed of passage (m · h–1 or m3·h–1·m–2)
t = temperature (°C)
A, K = experimental coefficients that are dependent on the biomass, support material (sand, biolite (see attached growth processes), GAC …) and of the technology used (especially according as to whether or not the reactor is aerated in situ).
nitrification utilisation
We have just discussed the two limiting factors in nitrification treatment:
- the availability of dissolved oxygen at the rate of 3.6 ppm of O2 to remove 1 ppm of NH4+; bearing in mind that we need to leave 1.5 to 2 ppm of O2 in the treated water, it is clear that water that has undergone aeration that provided it with 80% O2 saturation (frequently the case), we cannot remove much more than 1.5 to 2 ppm NH4+ through nitrification, at least without a further injection of oxygen; it can also be stated that for the same preliminary aeration technology, the nitrification capacity will increase if the temperature falls (oxygen solubility varying with temperature);
- nitrification kinetics where, according to equation (4), a drop in temperature, conversely, becomes unfavourable.
On a filtering bed, we may find the same apparent nitrification kinetics at (for instance) 5°C and at 30°C but for different reasons:
- less dissolved oxygen in hot water;
- slower reactions in cold water.
In practice:
- low NH4+ contents (ð 1 mg · L–1), found in well-aerated water, can be directly eliminated using this method through sand filters in the absence of any residual disinfectant (however, preliminary ozonation seems to encourage bacterial development); should maximum ammonium levels be likely to occur during cold water periods or if 1 mg · L–1< NH4+< 2, in order to increase the available biomass, we can replace sand with biolite having the same granulometry, thus forming a"nitrifying filtration bed" (see eliminating ammonium); there is also a floating bed variant (see filtrazur filters and eliminating ammonium);
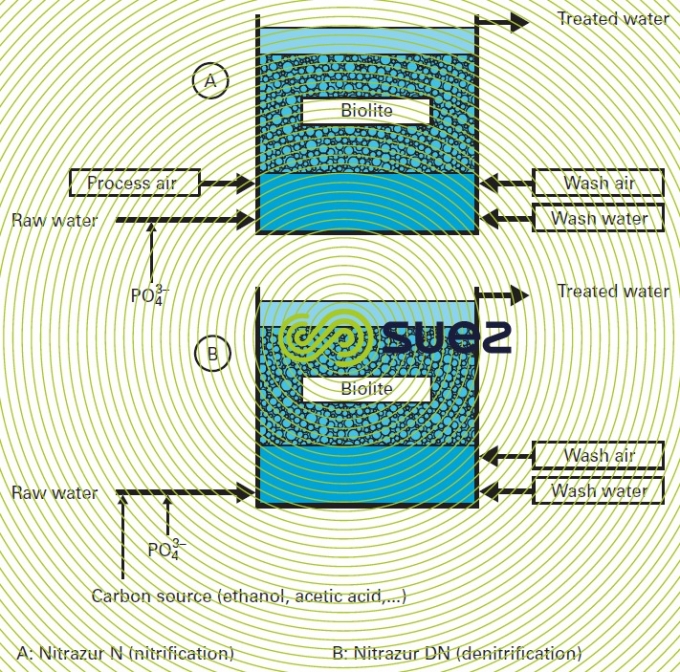
- nitrification of clean water having a high NH4+ content is used in aerated biofilters where water and air flow in co-current or counter current mode with a volume ratio [air (Nm3·h–1)/water (m3·h–1)] close to 1; theoretically, the biofilter’s granular mass can be made up of sand or of fine granulometry Pozzolana (see also eliminating ammonium); however the best performing machines are filled with biolite, e.g. the degremont® Nitrazur nitrificator (figure 43), a reactor similar to the Biofor Nused on wastewater and where air and water flow in co current mode. These reactors must be followed by filtration through sand.
denitrification
The biological process most widely used to remove nitrates uses heterotrophic bacteria which therefore have to be provided with a carbonaceous nutrient (most frequently, this will be ethanol).This is utilised thanks to the oxygen taken from the nitrates that are thus converted into nitrogen. These are facultative aerobic bacteria; the medium has to be deprived of dissolved oxygen for the nitrates to decompose. Additionally, as for nitrification, a source of phosphorus is essential.
This type of treatment is used in a biofilter that has a special granular material such as biolite. A more detailed description of the relevant Nitrazur DN process (similar to the Biofior DN) developed by SUEZ can be found in the section removal of nitrates Figure 44 sums up the main design differences between a Nitrazur N (see nitrogen conversion) and a Nitrazur DN filter.


Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later
















