the oxidants and disinfectants
Reading time:oygen
Oxygen is one of the most abundant elements found in Nature. In its molecular form O2, oxygen or "dioxygen" is a colourless gas that is relatively insoluble in water (see characteristic constants of gases).
As demonstrated by the oxidation reduction equations, the oxidising capacity of oxygen is heavily dependant on pH and low compared to other oxidants used. Thus, at neutral pH, the oxidation reduction capacity associated with oxygen rises to + 0.815 V. This shows that oxygen dissolved in water is only capable of dissolving certain compounds such as ferrous iron. Iron removal constitutes the main use made of oxygen as a chemical oxidant:


The higher the pH, the faster the reaction. One of the side-effects of open aeration used to oxygenate water is that this method will eliminate dissolved gases such as hydrogen sulphide H2S and carbon dioxide CO2 (see carbonate balance, Sedimentation).
In its fundamental triplet electronic state, oxygen acts like a bi-radical. It will then be chemically inert compared to the organic matter which explains why living organisms continue to exist. Three main techniques can be applied to exacerbate oxygen reactivity:
- excitation of oxygen in the singlet state through ultraviolet irradiation in the presence of photosensitisers (system applied in organic synthesis;
- the chelation of oxygen with transition metals (through oxygenase enzyme action for instance);
- oxygen reduction to a superoxide radical anion O2°– that is in a state of acid-base equilibrium with the hydroperoxyl radical HOO°, both species being capable of initiating a radical oxidation mechanism.
Only the two latter mechanisms are used in organic compound oxygenation reactions in water treatment applications. Spin-off processes are termed wet air oxydation processes (WAO) and are used at high temperatures and pressures in order to speed up the reaction. Depending on operating conditions and on the catalysts used, oxygen will then be capable of oxidising a range of organic compounds. This type of process is applied to the treatment of concentrated industrial effluent or to sludge treatment (see section organic matter destruction using the wet oxidation method).
chlorine and hypochlorite
Chlorine having the chemical formula Cℓ2 is a gas under normal temperature and pressure conditions.

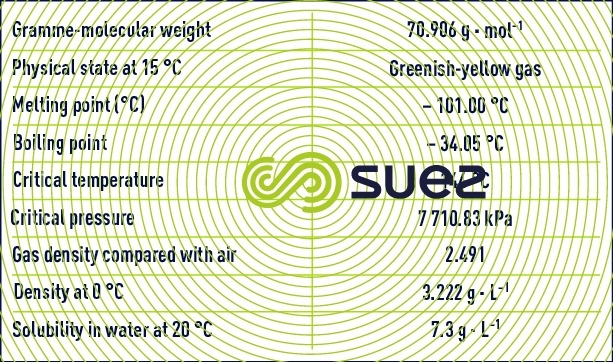

Chlorine injected into water dismutes very rapidly to produce hypochlorous acid and hydrochloric acid according to the endothermal reaction.


Chlorine hydrolysis takes a few tenths of a second to complete. It is followed by the dissociation of the hypochlorous acid.

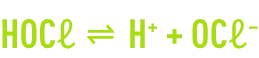
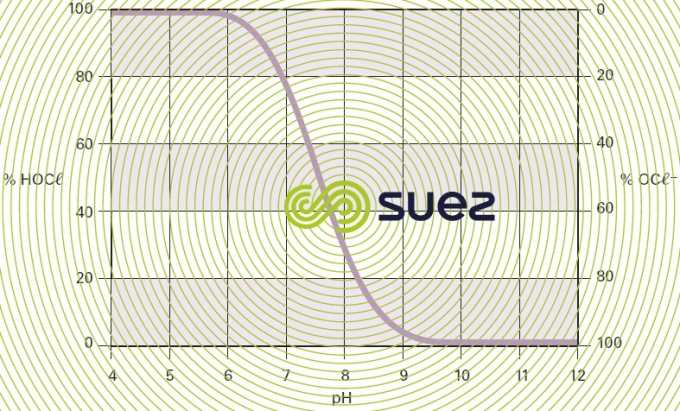
The dissolution of chlorine in water thus leads to the formation of hypochlorous acid and hypochlorite anions in varying proportions depending on pH and on the temperature of the medium. At 15°C, the relative concentrations of the two species will change with the pH as shown in figure 92.


Chlorine can be injected into the water as chlorine gas or as hypochlorite (sodium salt in solution (bleach) or calcium powder). The medium’s pH will then determine the nature of the species present. In practice, the following concentrations apply:
- available chlorine content, the sum of hypochlorous acid and hypochlorite determined by analysis;
- the available active chlorine content in the form of hypochlorous acid (subtracted from the available chlorine content using curves such as those shown in figure 92);
- the chlorometric degree of a hypochlorite solution, representing the concentration of a solution releasing one litre of chlorine gas under normal pressure and temperature conditions when hydrochloric acid is added; 1° chlorometric is the equivalent of 3.17 g · L–1 active chlorine;
- the active chlorine content expresses the equivalent concentration in hypochlorite-free chlorine; 1 g of sodium hypochlorite is the equivalent of 0.95 g of active chlorine.
Chlorine reacts with a range of substances in the form of hypochlorous acid and/or hypochlorite ion depending on the medium’s pH.
action on ammonia nitrogen

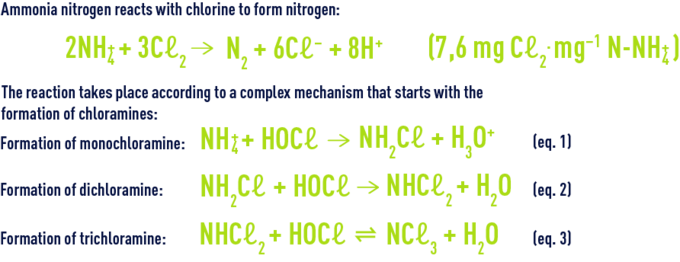
The organic and inorganic chloramines together constitute the chlorine termed bound chlorine as opposed to available chlorine. Nitrogen is released for a high level of chlorination. Nitrogen is produced by earlier reactions (hydrolysis, recombination, oxidation by chlorine) from mono and dichloramine. At neutral pH, monochloramine is the majority entity while the HOCℓ/NH4+ ratio remains below one. Monochloramine will be oxidised by chlorine according to the following reaction :

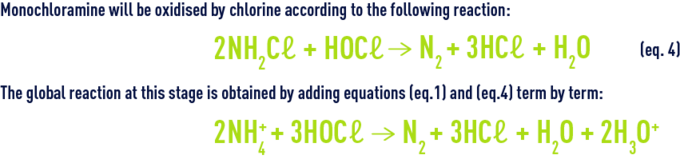
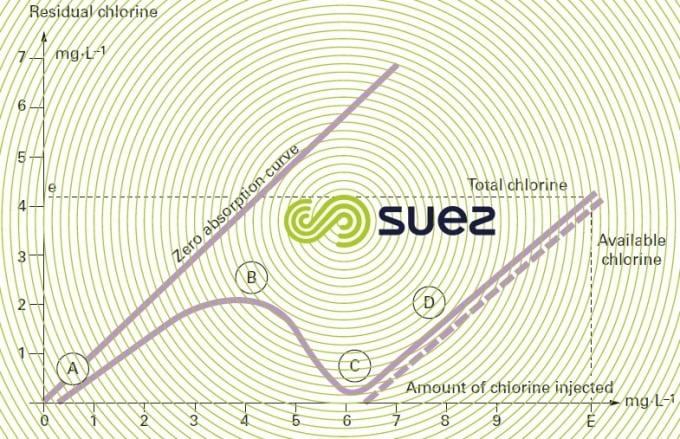
The amount of chlorine injected (which is the stoichiometric dose) is designated critical point or break point. On the chlorine absorption curve (figure 93), break point C marks the complete conversion (B to C) of the chloramines formed (A to B) and the start of available chlorine (D) appearance. When a natural water is chlorinated, the absorption curve will shift to the right: the presence of organic matter generates an immediate consumption of chlorine which shifts the break point beyond 10 mg · mg–1 of ammonia nitrogen.


action on other inorganic compounds
From inorganic pollution, chlorination can create compounds regarded as deleterious and subject to regulations (chloramines, bromate ion). The presence of these secondary products in chlorinated water mainly depends on the medium’s pH, on the amount of chlorine used and on the reaction time.



action on organic compounds
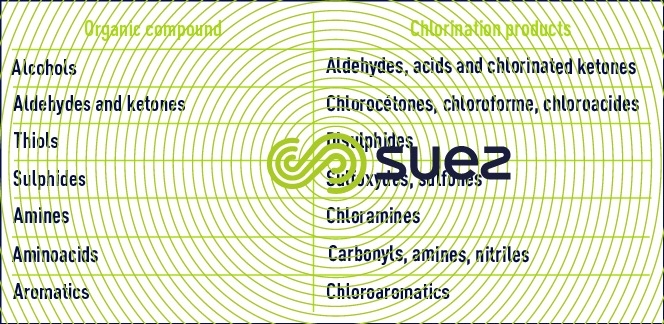
Chlorine has a relatively selective action on organic pollutants. Its reactivity involves privileged sites of attack (reducers, unsaturates, nucleophiles) and causes structural changes accompanied by the formation of more oxidised and/or replacement compounds (organochlorines). Therefore, water chlorination with the object of producing water for consumption can create compounds that are unpleasant from the taste/odour viewpoint (aldehydes, chlorophenols), toxic (trihalomethanes) and/or potentially carcinogenic (organohalogens) (see section pollution generated by water treatment). As in the case of inorganic compounds, the presence of these secondary products in chlorinated water will depend on the medium’s pH, the amount of chlorine used and the reaction time. These unwanted by-products mainly form in the presence of available chlorine and, therefore, at a treatment level above break point.


action on pathogenic organisms
Chlorine, especially as a hypochlorous acid, is a powerful biocide. Therefore, in order to achieve a good disinfection, we have to go beyond the break point. Chlorine has been recognised for its effectiveness as a bactericide and for its notable virulicidal action for inactivating pathogenic enteroviruses. However, it has a negligible effect on parasitic organisms such as encysted organisms (see section Oxydation et désinfection par le chlore).
The action of chlorine on ammonia nitrogen can be used to create chloramines that have a bacteriostatic effect because of their high remanence. Chloramines can be formed by chlorinating a water that contains ammonium ions below the break point or by simultaneously injecting chlorine and ammonium or ammonia salts with a Cℓ2/N-NH4+ mass ratio varying between 2 and 4.
chlorine dioxide
Chlorine dioxide is made up of chlorine that has a + IV degree of oxidation. The chlorine dioxide molecule has a paired electron and is regarded as a relatively stable radical.



Chlorine dioxide is extremely soluble in water. Its solubility depends on temperature and pressure. Its dissolution in water at a neutral pH results in a mixture of chlorous and chloric acids.


In a base medium, chlorine dioxide undergoes dismutation into chlorite and chlorate ions. This reaction is irreversible at pH levels above 11.

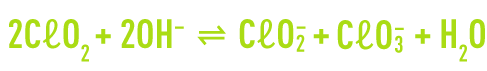
In a neutral or acidic medium, chlorine dioxide reacts slowly to form chloric acid.


Chlorine dioxide reacts more rapidly than chlorine with dissolved iron and manganese. It is also capable of oxidising sulphides, nitrites and cyanides. On the other hand, it will not oxidise bromides (except under simultaneous exposure to light), nor ammonia.
Additionally, chlorine present in water as hypochlorous acid will oxidise chlorine dioxide and the chlorite ion to produce chlorates.

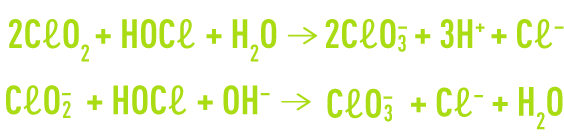



With regard to organic pollutants, chlorine dioxide has both a different chlorine reactivity and a narrower spectrum of action :
- chlorine dioxide reacts rapidly with phenol derivatives, tertiary and secondary amines, organosulphur compounds;
- chlorine dioxide is virtually inert with regard to unsaturated compounds other than phenolic compounds, oxygenated products, primary amines (amino acids).
Organic products derived from water treatment using chlorine dioxide are mainly carboxylic acids, glyoxal, aldehydes, ketones and, if applicable, polymers. During treatment, most of the chorine dioxide will be converted into chlorite ion whereas the residual chlorine dioxide will develop into chlorate and chloride ions. However, chlorites are toxic in the same way as nitrites (forming methemoglobin). That is why the total concentration of chlorine dioxide, chlorite and chlorate in water intended for human consumption is regulated in many countries and limitations are placed on the amounts that can be used.
Compared with the use of chlorine in water intended for human consumption, chlorine dioxide has the major advantage that it forms neither THM nor organohalogen (TOX) compounds. In practice, the excess chlorine used when generating chlorine dioxide still produces small amounts of TOX. Chlorine remains the preferred reagent for removing phenols, responsible for taste and odour problems; however, if not enough reagent is used, this can lead to the formation of organochlorine products that taste like medicine.
Chlorine dioxide is a more effective biocide than chlorine. It destroys viruses, bacteria and spores as well as biofilms in channels.
ozone
Ozone is a gas that is present naturally in the stratosphere as the result of the action of ultraviolet radiation emitted by the sun on oxygen molecules. Thus, it provides protection against harmful UV radiation.
As indicated by its chemical formula O3, ozone is made up of three atoms of oxygen.

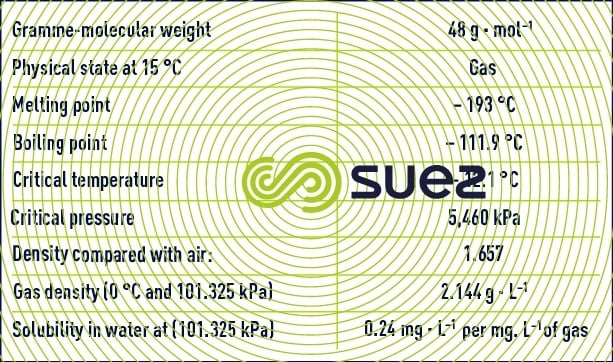

Ozone is not very soluble in water. Ozone solubility decreases as temperature rises or as the concentration of ozone in the gas diminishes (see section characteristic constants of gases).
As ozone breaks down in the water, a complex chain reaction mechanism occurs under the effect of the various solutes present in the water or released during treatment. This mechanism is :
- initiated by the hydroxide ion (high pH), hydrogen peroxide (formed during the oxidation of organic compounds), ferrous iron, formic and glyoxilic acids, humic matter;
- propagated by promoters such as aryl group compounds, formic and glyoxicil acids, primary and secondary alcohols, humic matter;
- and ended by inhibitors that consume the hydroxyl radical: carbonate and bicarbonate ions, high concentration phosphate ion, acetic acid, tertiary alcohols, alkyl group organic compounds, humic matter.
Therefore, the dissolved ozone’s lifetime depends on all the above parameters.
The chemistry of ozone in water is governed by two types of reaction: it can either act directly in its molecular form or it can be broken down by a range of mechanisms, resulting in the formation of the hydroxyl radical OH°, an oxidant that is even more powerful than ozone (E° = 2.80 V).
The oxidation of a substrate M can take place simultaneously via two reactions outlined in figure 94. However and in practice, the action of one of the oxidising species – hydroxyl radical or ozone – will predominate depending on medium conditions (mainly pH), the speed with which ozone reacts with the compounds present, the nature of the products formed (possibly capable of accelerating or slowing down ozone breakdown).

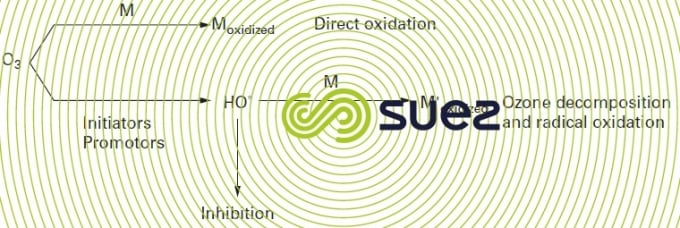

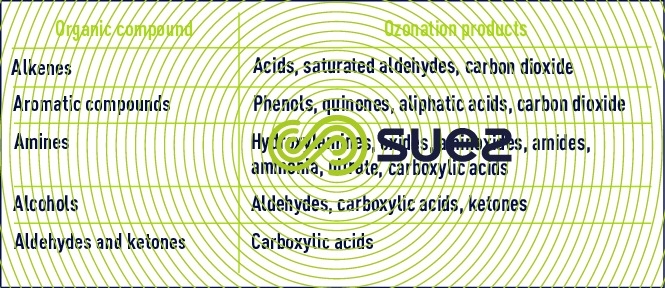
The different ways in which ozone reacts with organic compounds can be used to establish three categories of compounds:
- unsaturated aliphatics and aromatic compounds are easily oxidised;
- saturated and unsaturated oxygenated and halogenated compounds will be slightly degradable;
- compounds that have no C-H bond, such as tetrachloromethane or pentachlorophenol are totally inert.





Ozone is the most powerful chemical disinfectant used in water treatment. Additionally, its action on pathogenic agents is not pH-dependant. However, its poor stability in the medium means that the secondary use of a remanent oxidant becomes compulsory for distribution network safety purposes. Ozone’s ability to inactivate living cells can be extended to the point of provoquing their lysis. This property has been exploited as part of the Biolysis O treatment (see section reduced sludge production).
permanganate
Permanganate is a + VII oxidation degree oxygenated derivative of manganese. Permanganate is anionic and used as a potassium salt having the formula KmnO4.


This compound’s solubility in water increases with temperature. In the natural water treatment field, it will create two reactions depending on the medium's pH.
in an acid medium

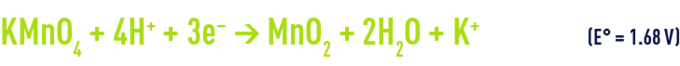
in a neutral or base medium

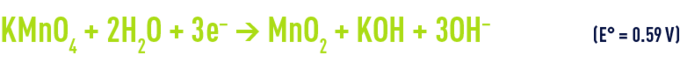
Permanganate reduction produces manganese dioxide that becomes insoluble at pH levels above 3.5. Therefore, the precipitated dioxide must subsequently be separated during a coagulation-clarification or filtration stage.
Permanganate reactivity with regard to pollution contained in water is heavily dependant on pH. In practice, we recommend operating at a pH of between 6 and 8.5. Under these conditions, the inorganic pollutants affected by the permanganate will mainly be dissolved iron and manganese. Sulphides and cyanides may also be oxidised. However, ammonia nitrogen and bromide ions undergo virtually no conversions.




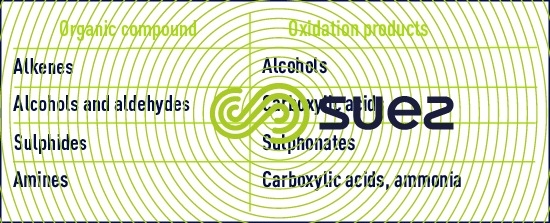

Organic compound oxidation by permanganate involves complex mechanisms. Reactivity follows the sequence shown below :
- sulphur compounds, primary amines and aldehydes react quickly;
- ketones and aromatic compounds are converted slowly.
The use of permanganate does not lead to the formation of trihalomethanes.
Finally, it is accepted that the action of permanganate is not enough to thoroughly disinfect drinking water. Post-disinfection is required.
hydrogen peroxide
Hydrogen peroxide has the chemical formula H2O2.

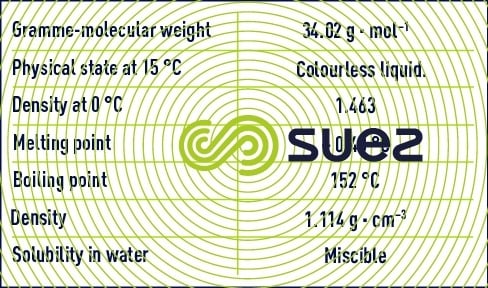

Hydrogen peroxide is a metastable compound. It breaks down easily into oxygen using an exothermal process.

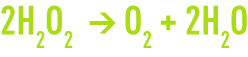
The breakdown occurs under the effect of numerous porous substances (such as pumice stone, platinum foam, manganese dioxide) or of dissolved substances (such as hydroxide ions and some metals). This reaction will be more or less violent depending on the strength of the peroxide solution.
Hydrogen peroxide is available as a 30% to 70% by weight water-based solution. Solution concentration is often expressed as a volume that corresponds to the volume of oxygen that can be released by one litre of solution and measured under normal temperature and pressure conditions (0°C and 101.325 kPa). The weight percentage is converted into volume as follows:
- volume = 3,67 × % massique (exemple 30 % en masse égale 110 volumes).
Hydrogen peroxide’s reactivity in water is linked to its weak acid nature and to its oxidising and reducing capacity.

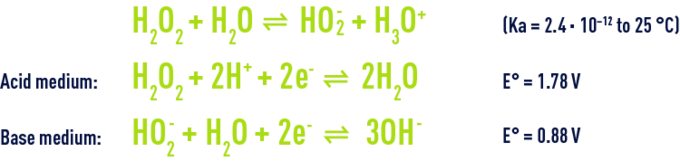
At natural water pH, hydrogen peroxide is present in water in its molecular form. It can react with pollutants as summarised in table 37.



Hydrogen peroxide is not used much in water treatment. Its application is reserved to a few specific actions that are primarily designed to remove sulphides and cyanides.
Similarly, its bactericidal and algicidal properties are only used in water treatment systems applicable to a few swimming pool and industrial circuit water cases.
paracetic acid
Paracetic acid is also called peroxyacetic acid or ethanoperoxoic acid and, like hydrogen peroxide, belongs to the group of peroxide compounds identified by the presence of two adjacent atoms of oxygen (-O-O-, "peroxy" group). Its chemical formula is written CH3COOOH.
Paracetic acid is a colourless liquid with a sharp odour and can be mixed with water in any proportion. This compound is unstable and does not exist in a pure state. It is available as an aqueous solution mixed with acetic acid and hydrogen peroxide according to the following equilibrium:

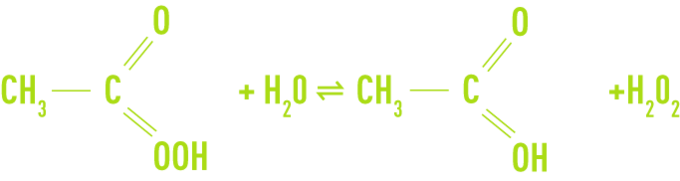
In addition to paracetic acid, acetic acid, hydrogen peroxide and water, commercial solutions contain acid type chelating stabilisers (sulphuric acid). The most highly concentrated solutions contain 40% of paracetic acid, 40% of acetic acid, 5% of hydrogen peroxide and 13% water.

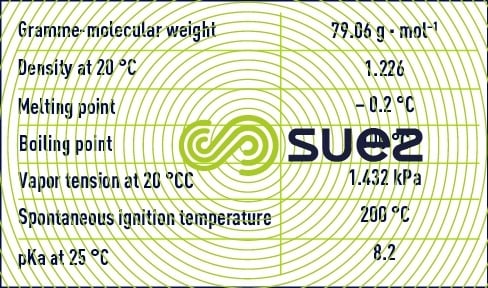

Paracetic acid in an aqueous solution is less stable than hydrogen peroxide. Paracetic acid will break down spontaneously to form acetic acid and oxygen. This reaction is speeded up by heat (explodes when heated to 110°C) and in the presence of metal ions (iron, copper, manganese, nickel, chromium...).


Paracetic acid is a powerful oxidant capable of reacting with many organic and inorganic compounds. The following constitute the main uses made of its oxidising properties:
- chemical synthesis (polymerisation initiator and reticulation agent);
- for bleaching textile fibres and paper pulp;
- disinfection and sterilisation in the agri-food industry and in the hospital sector.
The indicator response (total coliforms, E. coli, faecal streptococci, Clostridium spores, bacteriophages) to paracetic acid’s anti-bacterial action means that it can be considered for use in waste water disinfection, especially in cases of temporary disinfection (a few months per year).
UV radiation
The term ultraviolet radiation applies to radiations in the electromagnetic spectrum having wavelengths between visible and X-rays, i.e. between 100 and 400 nanometres. The UV spectrum itself is divided into four wavelength groups (figure 95).

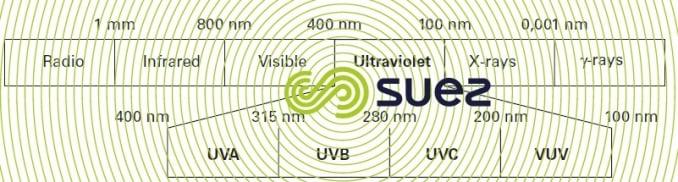

The entire UV domain is the centre for waves designated actinic or chemical because the photon energy emitted is capable of generating chemical transformations via a photochemical process. Radiation used for disinfection purposes comes within the UVC spectrum that defines the germicidal range. In effect, cell, protein and nucleic acid materials absorb light between 200 and 300 nm with maximum absorbance at 260 nm in the case of DNA.
This UV radiation is produced using low or medium pressure mercury vapor or lamps or lamps with quartz covers. Depending on the case, emission will be almost monochromatic at 254 nm or polychromatic (see ultraviolet disinfection).
The germicidal mechanism is based on photon absorption by DNA pyrimidine bases, mainly thymine but also cytosine. Irradiation then induces dimerisation starting from the adjacent bases, fracturing the DNA chain in such a way that subsequent replication is inhibited (figure 96). UV radiation effectiveness has been demonstrated in the elimination of bacteria whether or not spore forming, rotovirus and poliovirus and protozoa cysts such as Cryptosporidium cysts. However, helminth eggs will not be inactivated.

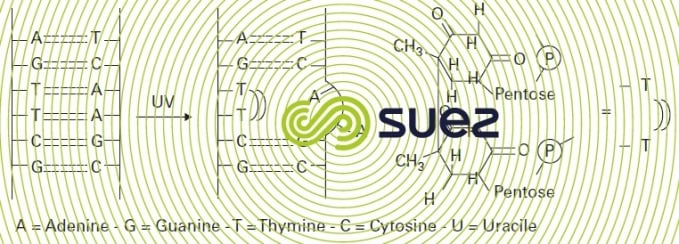

The quality of disinfection may be limited by the regrowth of some micro-organisms. This problem is linked to the development of mechanisms that are capable of regenerating the DNA structure:
- photoreactivation (pyrimidine dimer monomerisation);
- excision-repair (replacing damaged nucleotides and recombining patterns that have not been altered).
Studies carried out have shown that photoreactivation is possible for total and faecal Coliform, Escherichia Coli, Streptomyces, Aerobacter, Penicillium, Saccharomyces and Micrococcus germs.
UV radiation applied as part of disinfection treatment is recognised for its production of a minimum of by-products. The chemical compounds present in water that absorb wavelengths emitted for disinfection purposes can, however, generate by-products. This phenomenon increases as the light spectrum specific to the UV production system widens compared with the germicidal wavelength (case of medium pressure lamps).
This phenomenon mainly involves aromatic core compounds and chlorinated aliphatic compounds; however, their photo-oxidation requires extremely high doses of irradiation that have no relevance to normal application conditions. At very high dosage levels, the formation of assimilable organic compounds and the development of mutagenicity are highlighted as the result of the irradiation of organic compounds dissolved in natural water that absorb light between 200 and 250 nm.
Similarly, the nitrites produced by the photolysis of nitrates appear at wavelengths below 240 nm.
advanced oxidation systems
In order to further improve chemical oxidation processes, so-called advanced oxidation processes have now been developed with the ability of generating high oxidation performances in the case of organic pollutants found in water. They share the principle whereby they are capable of generating a more powerful and less selective secondary oxidant in the reaction medium by activating an available primary oxidant. In most cases, the reactive species formed will be the hydroxyl radical whose standard oxidising reduction potential reaches 2.8V at 25°C.

This secondary oxidant can encourage the oxidation of most organic compounds until they are fully mineralised as carbon dioxide and water. The hydroxyl radical usually reacts at least one million times faster than ozone or hydrogen peroxide, thus leading to smaller plant sizes.
There are many advanced oxidation processes. They are classified by primary oxidant activation type:
- chemical activation in the case of processes involving the ozone/hydrogen peroxide pair,


- photochemical activationin the case of processes based on ultraviolet irradiation combined with ozone and/or hydrogen peroxide oxidants or involving a semiconductor type photocatalyst such as titanium dioxide,

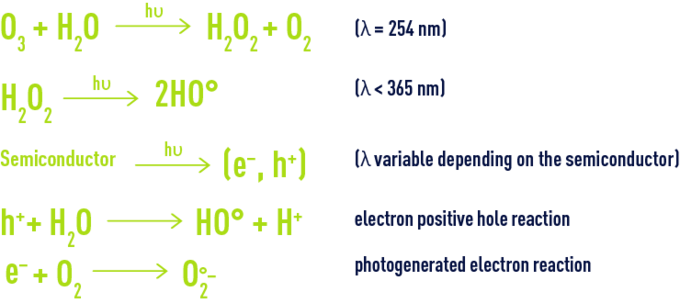
- catalytic activation, with the earliest process developed and which is the Fenton system (ferrous iron/hydrogen peroxide), the catalytic ozonation processes including the Toccata (see section catalytic ozonation : toccata) and a catalytic process based on hypochlorite.

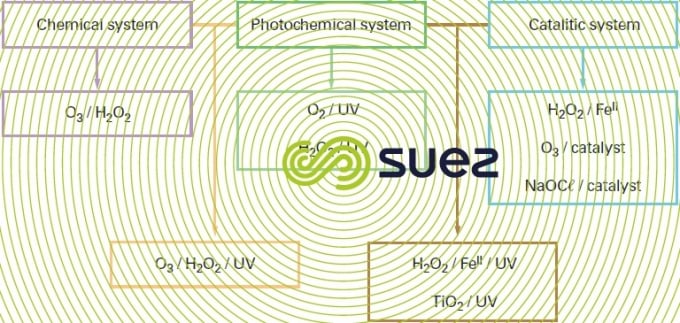

Since their introduction onto the market in the 1970s, advanced oxidation process installations have only gradually entered into water system applications. The combined O3/H2O2 system is the one that is the most widely used, especially for the purpose of eliminating pesticides with a view to producing water intended for human consumption. The processes involving this method are no longer approved for use in France due to by-products (bromates and pesticide oxidation products) whose formation they encourage. Photochemical processes tend to be reserved for the removal of low concentration organic compounds when re-instating groundwater, for instance. Catalytic processes have a range of activity that extends to the treatment of industrial wastewater produced by papermaking, textile and chemical industries… (see section catalytic ozonation : toccata).
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












