removing hardness (calcium and magnesium)
Reading time:main methods
lime used to remove carbonates
basic reactions
The following carbonate removal reactions apply:

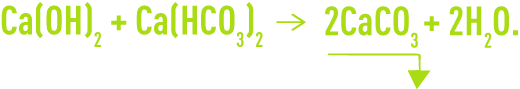
However, as in most cases water contains free CO2, this will be the first to react with the lime to produce hydrogen carbonate:


initially resulting in increased M-alk. and CaH before reaction (1) takes over.
When CaH < M-alk. (water with calcium and magnesium alkalinity), we then have:

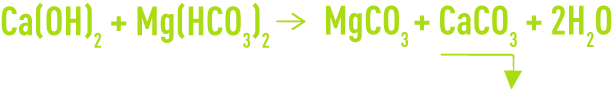
As magnesium carbonate is relatively soluble (approximately 70 mg·L–1), an excess of lime will lead to the following reaction:

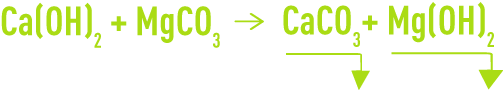
And if amounts of reagent are precisely adjusted, the water’s alkalinity will be reduced to the theoretical solubility applicable to the CaCO3 + Mg (OH)2 system which ranges from 2 to 3°F under normal temperature and concentration conditions.
However, this limit M-alk. figure may be increased in practice when dissolved impurities are present (e.g. organic acids, ammonium, phosphates, etc.).
As indicated by the above equations, these reactions will only eliminate bicarbonate hardness (TH balanced by some M-alk.). Therefore, these reactions will eliminate neither permanent hardness (TH – M-alk. > 0) that requires the addition of sodium carbonate nor those bicarbonates that are not hardness-related (water with sodium alkalinity) that require added excess lime.
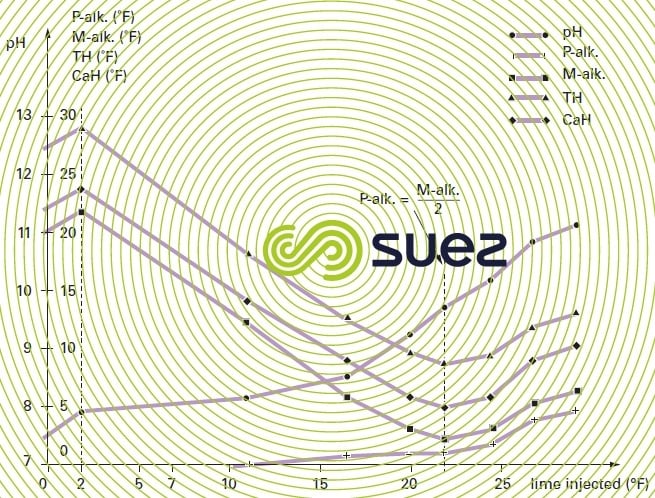
Using the example of water with bicarbonate alkalinity, figure 5 recaps the changes in the various titers based on the amount of lime added.


crystallisation mechanism
The calcium carbonate formed will be crystalline and, accordingly, have precipitation kinetics that obey the germination-crystallisation laws, viz:
- in the absence of crystallisation seeds, precipitation will be very slow, water will remain supersaturated and precipitation of the few suspended substances will tend to take place but mainly over available surfaces, especially if they are metal surfaces (reactor walls, agitators, channels, valves…). We will then observe a gradual but often quick scaling take place;
- conversely, in the presence of crystals, precipitation takes place very fast and a balance is achieved within a few minutes. This applies at least when the surface of the available crystals remains sufficiently "clean": no impurities such as adsorbed organic polymers, colloids, metal hydroxides. In particular, when Mg(OH)2 is precipitated, it will tend to slow precipitation down and above all to significantly lighten the crystal aggregates that have formed. Therefore, the reactor and separator used will have to be bigger, see chapter Flocculators – settling tanks – flotation units.
A good carbonate removal unit must, therefore, include:
- a zone where recirculated crystals, water to be treated and lime are thoroughly combined;
- a settling zone from which the crystals that have been formed are removed and partially returned to the 1st zone.
A coagulant and/or flocculent will be required in order to improve the sedimentation rate and to allow the use of a rapid lamellar type settler (Densadeg type with rates of > 30 m · h–1).
using sodium carbonate
Removal of permanent hardness is carried out cold with sodium carbonate which may or may not be combined with calcium and magnesium bicarbonate precipitation using lime. This elimination takes place according to the following reactions:

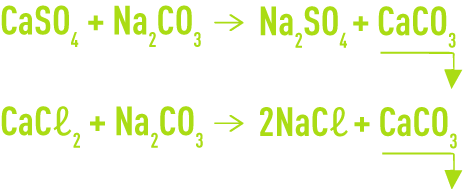
However, this process does not allow us to reduce M-alk. to below 3 or 4 °F in the best cases.
precipitation using caustic soda
Calcium and magnesium ions removal through precipitation with caustic soda constitutes a variant of the combined lime and sodium carbonate process described under.
The following constitutes the basic reaction:


Calcium carbonate precipitation takes place with the formation of sodium carbonate that will react with permanent hardness according to reactions (5) and (6) above.
Using caustic soda will, therefore, lower water hardness to a level that is equal to twice the reduction in bicarbonates belonging to the alkaline-earths. The water’s M-alk. can only be lowered to approximately 3 or 4°F if there is sufficient permanent hardness available to combine with the sodium carbonate formed. Therefore, the use of sodium hydroxide is only recommended in this case; however, it may also be considered for the partial softening of water where CaH > M-alk.
precipitation calculation and control (obtaining a minimum M-alk.)
We refer to:
- CaH calcium hardness in °F referring to total calcium content;
- MgH magnesium hardness in °F referring to total magnesium content;
- C the free CO2 content expressed in °F:

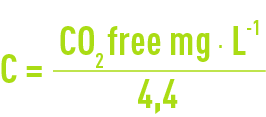
amount of lime
positive TH-M-alk.
The theoretical amount of lime required for optimum precipitation of calcium carbonate will be as follows according to reaction (1):

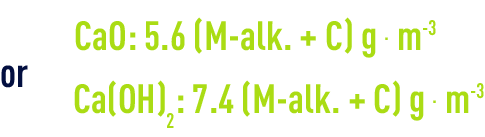
If we have to precipitate both calcium carbonate and magnesium hydroxide (i.e. the Mg linked to the M-alk.), as MgH will be greater than TH-M-alk. (Mg(CO3H)2 titer = M-alk.-CaH), the following amount of lime will be needed:

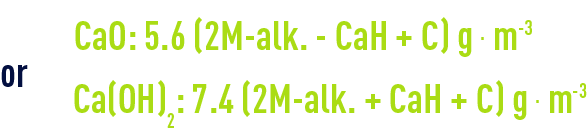
negative TH-M-alk.
This concerns water with sodium alkalinity. We can always achieve satisfactory calcium and magnesium precipitation by calculating lime on M-alk. + C. However, this method will produce water that is loaded with caustic soda and it may be advisable to use smaller amounts. This comes down to only precipitating CaCO3 in order to remove the ion that causes the greatest scaling:

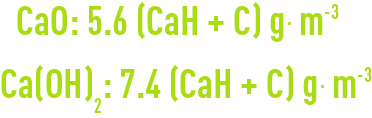
checking results
In all cases, we have to increase (or reduce) the amount of lime used by 5.6 g per m³ (CaO) or by 7.4 g per m³ (Ca(OH)2) for each degree of P-alk. recorded above or below the theoretical rule.
Clearly, figures 5.6 and 7.4. apply to 100% products. In reality, lime will always be impure and more or less carbonated and industrial figures need to be increased by 10 to 30% depending on the situation. If we limit ourselves to precipitating calcium carbonate, the ideal setting will be obtained for:

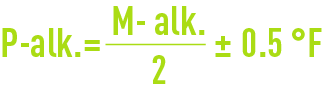
which setting refers to a minimum M-alk. of approximately 2°F when the water does not contain any magnesium or crystallisation inhibiting compounds.
When MgH is greater than TH-M-alk., the application of this rule will lead to excessively high M-alk. values because of magnesium carbonate solubility and the optimum result will be obtained when :

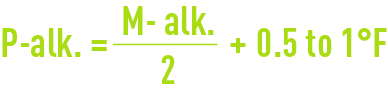
with a P-alk. value that is as low as possible.
amount of sodium carbonate required
The following amount of pure sodium carbonate will be required:

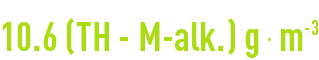
checking results
Theoretically, we should obtain the following for a magnesium-free water:

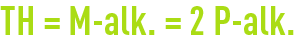
In practice, if we are treating water where the permanent hardness is partly due to magnesium, this rule may be ignored and the problem examined on a case-by-case basis.
amount of sodium hydroxide required
The following amount of pure caustic soda will be required:

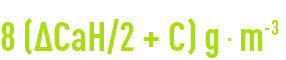
checking results
To lower the M-alk. by 1°F, we need to lower the TH by 2°F.
In practice, the amount of caustic soda injected into water must be adjusted so that we obtain a minimum amount of residual M-alk..
special case of partial carbonate removal
If we are only aiming for a partial reduction (e.g. storm water) of calcium hardness, designated by D CaH, the amount of lime used will be based on C + D CaH (or C + D M-alk.). When the reagent used is sodium hydroxide, the above calculation applies:


Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later













