treatment objectives
Reading time:The aim for any water delivered by a treatment plant is that it should be close to equilibrium i.e. neither aggressive nor scale-forming.
In order to guarantee the above condition while complying with pH values that are compatible with water potability (pH <8.5) or with the dissolution of other elements (e.g. lead at pH <7.5), we are obliged to produce a water that has a pHs between 7.5 and 8.5, i.e. alkalinity ranging between 8 and 20°F for hard water,, consequently, water that is neither too soft nor too hard.
Most of the treatments that we will describe in reagent and treatments than can be used concern soft water that requires remineralisation; however, the problems generated by water that is too hard (scale-forming/dissolving lead) can cause the water treatment specialist to reduce water mineralisation by carbonate removal and/or softening; the typical objectives to reach are:

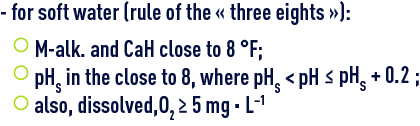
These conditions are necessary for the development of a protective carbonaceous layer on the steel walls or cast iron pipes; this layer consists of a mixture of CaCO3 (calcite), FeCO3 (siderite), FeOOH (goethite), Fe3O4 (magnetite), that is sufficiently isolating and that will inhibit the tendency of these metals to corrode in the presence of water (see natural water used in carbon steel passivation) ;

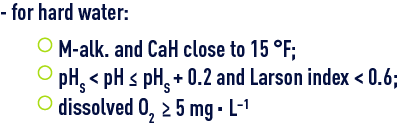


Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












