mechanism
Reading time:Adsorption defines the property of some materials, more or less reversibly, fixing ions or molecules (gases, metals, organic molecules…) on their surfaces. Matter is transferred from the aqueous or gas phase towards the solid surface.
The capacity for adsorbing a solid will depend on :
- the developed surface area or specific surface area (m2·g–1) of the material. Some solids found in the natural environment (clays, silica …) have very high specific surface areas that vary with the physical-chemical state of the aqueous medium (pH, nature of bonded cations). Thus, some clays such as the bentonites (Montmorillonite, for instance) have a surface that is accessible to most molecules and that varies from 40 to 100 m2·g–1. These materials have an extremely variable adsorption capability but constitute a parameter that is essential to the regulation of exchanges and to the mobility of elements in the natural environment.
Industrial adsorbents (mainly activated carbons) develop significantly greater specific surface areas (600 to approximately 2 500 m2·g–1), indicative of an extremely high microporosity. Other adsorbents such as metal hydroxides formed during the coagulation-flocculation process, also develop extremely large surface areas whose extension is closely linked to the pH;
- on the nature of the adsorbate-adsorbent bond, i.e. G interaction-exempt energy between the adsorption sited and the part of the molecule that is in contact with the surface. This energy can be measured direct in the case of gas adsorption. However, in an aqueous medium, calorimetric techniques will only measure the adsorption differential enthalpy for the difference between adsorbed molecule adsorption energy on the one hand and the desorption energy of the water associated with the interface, on the other. In the main, the Van der Waals active forces and the electrostatic forces (Coulomb) cause the adsorption. For example, we can observe that aromatic molecules have a strong affinity with the graphitic structure of carbon and will repel non aromatic polar molecules;
- the contact time between the adsorbant solid and the solutes: this is the time allowed to enable pollutants to migrate to the surface of the carbon.
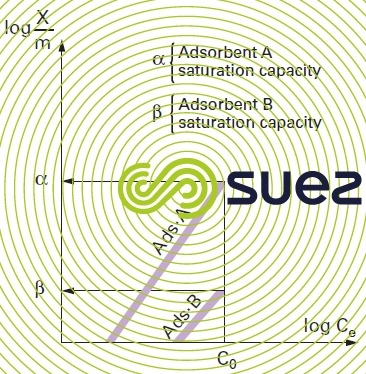
The mass of pollutant adsorbed per unit of adsorbant mass depends on the pollutant concentration in the aqueous phase. In the state of equilibrium, a dynamic exchange takes place between the molecules of the adsorbed phase and those remaining in solution. Many theories have attempted to model the relationship that exists between the quantity of molecules adsorbed (g · g–1 or g · m–2) and the quantity remaining the aqueous phase in the state of equilibrium. One of the most widely used theories in the field of adsorption over activated carbon is the Freundlich law that states:

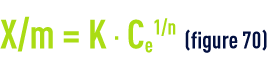
Where :
- X/m is the mass of solute fixed per unit of adsorbent mass;
- Ce is the pollution molecule concentration in the aqueous phase in the state of equilibrium after adsorption;
- K and n are energy constants based on the adsorbate/adsorbent pair at a given temperature that is kept constant throughout the experiment (hence the designation isotherms given to the graphs concerned). In fact, no modelling, however "complex", can be used to cover the entire isotherm and even less to explain the adsorption mechanisms. The main reason is that the entire surface area is physically and energetically heterogeneous.


Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












