the calcium-carbonate balance
Reading time:general: natural water
As discussed in the sections Water’s chemistry, Terms used by water analysts and Natural water, it is the geological structure of the ground that collects, drains and stores water and that helps to determine the water’s "appearance".
Low solubility granite structures are associated with water that has a low salt content but that is likely to contain a lot of CO2 (depending on the quality of the upper layers); conversely, alluvial or karstic areas will produce water with high ion concentrations, especially Ca2+, Mg2+ and HCO3– ions. As it flows on the surface (rivers-lakes), water enters into an equilibrium with the atmosphere, receives pollution… all of which contributes to the gradual change in its original properties.
soft water/hard water
As a consequence of the above, water hardness, measured by its TH (in F°) will vary widely from one area to the next (tables 43 and 44).
For example, water that has a TH < 5 °F will be termed "soft", and "hard" from a TH of between 15 and 20°C.

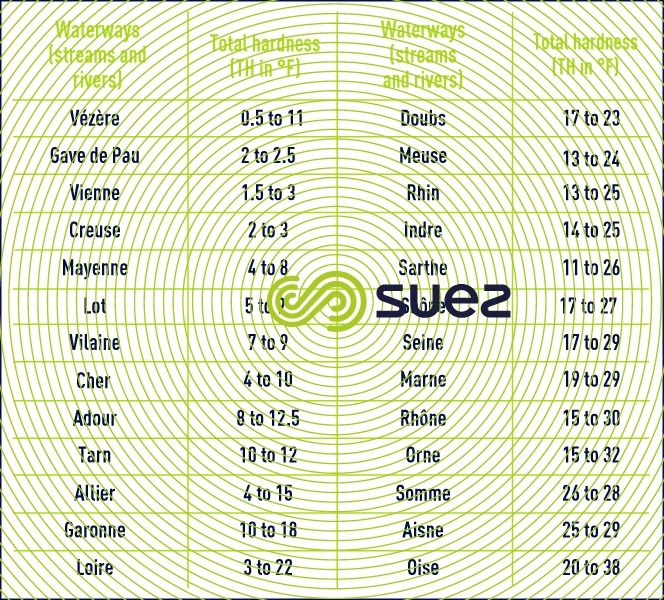

Throughout the world:



aggressive water/scaling water
Before we even embark on a theoretical interpretation of calcium carbonate dissolution/precipitation phenomena, it is quite logical to deduce that water with low salt contents will have retained a dissolution capacity with regard to the materials they come into contact with (recipients, pipes …).
Conversely, water with high salt contents and especially salts belonging to the alkaline earths, can deposit the less soluble of these salts; they will tend to create deposits that result in the formation of crystals at the solid-liquid interface.
A particular feature of calcium bicarbonate is that it only exists in the dissolved form; when it precipitates, it does so in the calcium carbonate form, one that is only slightly soluble and it can only remain in water when it is balanced out by available CO2:


As the CO2 content has an influence on pH, we can conclude that, for a given mineralisation (fixed CaH and M-alk.), there will be a pH value for the equilibrium between this water and the calcium carbonate: this value is termed the Langelier pHor saturation pH hence the designation "pHS" (the corresponding CO2 content then being know as the balancing CO2, a concept that will be quantified further on).
Therefore, we can "classify" water on the basis of its pH compared with this pHS:
- if pH > pHS, water will tend to deposit CaCO3 and is then designated as scale-forming;
- if pH < pHS, water will tend to dissolve CaCO3 and is then termed aggressive.
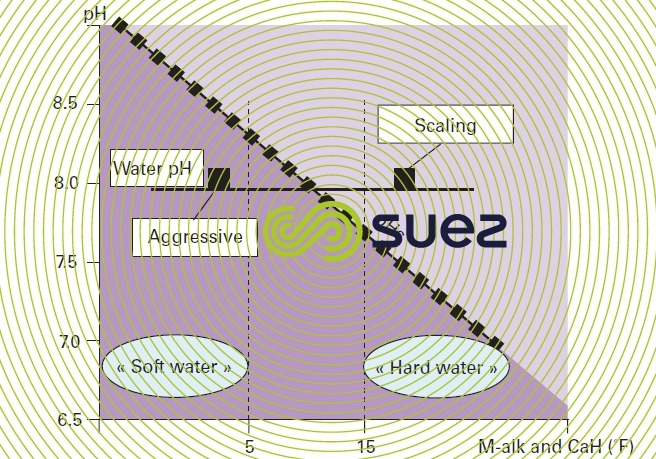
Note: later on, we shall see that the pHs value of water rises as its M-alk. (and, therefore, its mineralisation) decreases. E.g. (figure 99) for a pH that is systematically set to 8 (discharge into a mains system), lightly mineralised water will have a higher pHs and will, therefore, still be aggressive; on the other hand, a more mineralised water will have a lower pHs and will, therefore, be scale-forming; this explains the degree of "correlation" that links soft water with aggressive water, or hard water with scale-forming water, despite different meanings.
Carbonate calcium potential precipitation (CCPP) is an interesting parameter for quantifying what may be scale-forming or may have dissolving properties (pipes made from concrete) knowing that the CCPP is a level of concentration expressed in CaCO3 (positive if water can cause scaling or negative if water is aggressive).


Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












