theoretical bases for gas/liquid exchanges
Reading time:The following constitute the main laws governing gas/liquid exchanges :
- din the liquid phase and at equilibrium, the Henry law that links, for a given temperature, the partial pressure of a gas p to its molar fraction x in liquid phase p = Hx, H being the Henry constant. Please refer to section characteristic constants of gases, for the Henry constant applicable to the main gases ;
- in the gas phase: Dalton’s law and the ideal gas law.
Thus, for a gas mixture occupying a volume V at a temperature T and a pressure P and constituted by m1, m2… mn quantities by mass of gases having gramme-molecular weight of Ml, M2 Mn respectively, applying partial pressures p1, p2 pn, we can write :

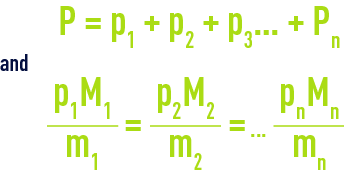
For the transfer : the Whitman & Lewis theory used to quantify the global stream N of the compound transferred through the exchange surface S in the absence of any accumulations at the interface (figure 108) :

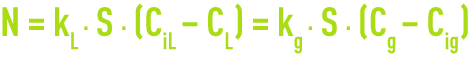
CL and Cg are the gas concentrations in the liquid and gas phases that are only accessible to measurement.
CiL, and Cig. are the concentrations at the interface, kL and kg being the transfer coefficients in the liquid and gas phases that are dependant on the interface and on turbulence conditions.

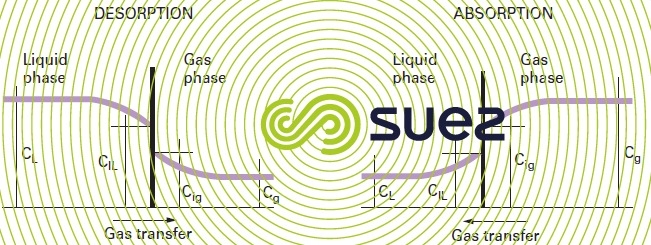

These laws highlight the parameters that are essential to an efficient transfer :
- maintaining a high concentration gradient between the liquid and the gas phase, this gradient acting as a driving force;
- creating a gas/liquid interface that is as large as possible;
- using high levels of turbulence for each of these 2 phases.
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












