water treatment applications
Reading time:electrochlorination
Electrochlorination is a technique used to produce, in situ, diluted sodium hypochlorite from a sodium chloride solution (seawater or brackish water).
reactions
The following global reaction summarises the way in which hypochlorite is formed from chloride :


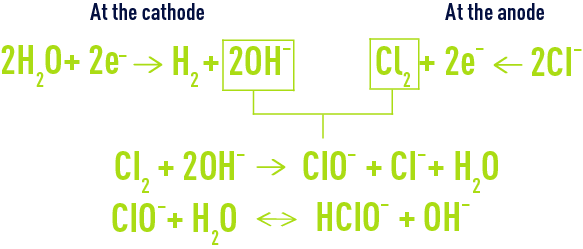
in fact, the following reactions are involved.
main electrochemical reactions

parasitic reactions

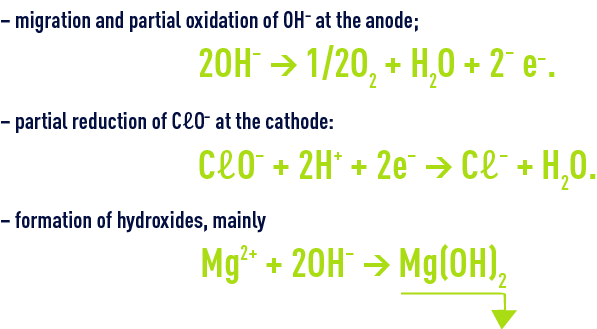
The first 2 reactions reduce electrochlorination efficiency; the last reaction is typical of electrochlorination using seawater and causes the electrodes to scale up; this phenomenon has to be overcome by means of regular acid washes.
areas of application
Hypochlorite, an oxidant-disinfectant, is frequently used in water treatment. From a security and procurement viewpoint, when electrochlorination is used to generate hypochlorite in situ, this abolishes the constraints created by the need to store chlorine or to transport and store dilute hypochlorite solutions (see section chlorine).
Therefore, electrochlorination was primarily developed in order to protect cooling circuits found on offshore platforms, in electricity generating stations or in factories supplied with seawater, against the proliferation of algae and molluscs but also in swimming pools…
Industrial units consume approximately 4 kWh per kilogram of chlorine equivalent produced. The concentration levels in the hypochlorite solutions produced tend to vary from 1 to 3 chlorine-equivalent per litre.
metal recovery
In many industries, but mainly those involved in surface treatment, hydrometallurgy and electronics, electrolysis is used to extract metals (Cu, Zn, Ni, Au…) from various solutions and, therefore, to reuse these metals.
electrocoagulation
This electrochemical treatment is used on some wastewater and is primarily responsible for coagulation-flocculation based on the following processes :
- creation of an electrical field between electrodes, encouraging collision between charges present in the effluent ;
- release of metal ions (Fe, Aℓ) by dissolving soluble anodes; as these ions are thoroughly dispersed throughout the fluid, they initiate the coagulation-flocculation mechanisms described earlier (see section different types of sedimentation)
The amounts of energy consumed by this process will vary from one application to the next but will often be in the region of 2 to 4 kWh per m³ treated.
other applications
- Electroflotation: this process generates oxygen and hydrogen microbubbles by electrolysing the water causing water to be floated (very rarely used).
- Electrodialysis: see section permeation processes.
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












