typical reagents
Reading time:Coagulation and flocculation reagents are simple or polymerised mineral salts, and organic, natural or artificial polymers. Tables in ain reagents used in water treatment and ain reagents used in water treatment summarise the properties of the commercially available forms of the different reagents.
mineral coagulants
The reactions described in this paragraph are summarised versions of the various reactions involved. All the products formed are soluble with the exception of Aℓ(OH) 3 and Fe (OH) 3.
Because of:
- their harmlessness (sometimes questioned in some countries with regard to aluminium: the, as yet unproven, part they play in Alzheimer disease);
- effectiveness (trivalent cations) with a minimum solubility in the region of a pH 7;
- cost.
The main coagulants used are based on aluminium or iron salts. In some cases, synthetic products such as cationic polyelectrolytes (described further on) can also be used.
aluminium salts
simple salts
The basic reaction, when the Aℓ3+ ion is added to water, consists of the formation of an aluminium hydroxide precipitate and the release of a degree of acidity (hydrolysis):

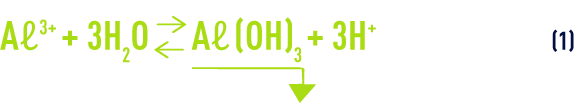
This acidity reacts with certain species in solution, especially bicarbonate ions (hydrogen carbonates):

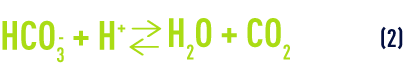
The overall reaction is:

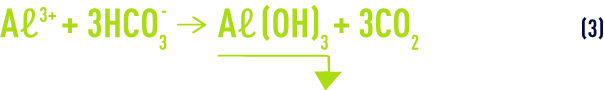
If the raw water’s buffering capacity ( M-alk. ) is not sufficient, this acidity must be compensated when adding the coagulant by also adding a base (sodium hydroxide, lime, sodium carbonate).
In fact, these are only overall reactions; the actual mechanisms are more complex and will involve forms that are halfway between the Aℓ3+ ion and hydroxide Aℓ(OH)3, in the following form:


E.g. Aℓ(OH)2+, Aℓ(OH)2+
These ions can also hydrate themselves, according to the following general formula:

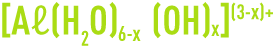
Such complex ions then undergo polymerisation through an olation reaction that consists in the bridging of OH hydroxyls:

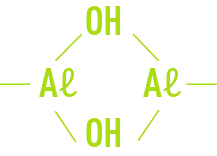
The diversity of polymers likely to form according to local conditions (pH, temperature, dissolved salts, quantity and nature of colloids…), ranges from


(which refers to the general formula


,which explains the differences in flocculation found between one water and another.
Obviously, these phenomena are not independent of those applicable to the neutralisation of the suspended solids loading in raw water: in fact, these are complex ions that tend to migrate to the surface of the particles, generating turbidity, allowing these particles to be thoroughly mixed into the floc as it forms; therefore, floc formation can be regarded as hydroxo-aluminous complexes forming bridges between the initial colloids found in raw water.
The most common reagents used are:
- aluminium sulphate, either crystallised: Aℓ2(SO4)3, 14 or 18 H2O, or liquid: 600 or 720 g · L–1 solution of crystallised aluminium sulphate 18H2O; commonly called "Alum" in English.
- liquid aluminium chloride AℓCℓ3(effective but rare)
- liquid aluminate Aℓ2O3, NaOH which in fact hydrolyses to produce AℓO–accompanied by more or less excess NaOH.
As already explained, when sulphates, chlorides or even Aℓ sulphate chloride are hydrolysed, this process releases acidity that needs to be neutralised by breaking down bicarbonates. The pH will fall more or less rapidly (outside the optimum pH zone…) depending on the water’s buffer capability, according to the global reaction (e.g. with sulphate, the most common case):

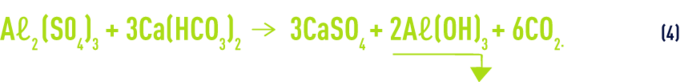
From this, we can deduce the following:
10 mg · L–1 of commercially available alum 18 H2O (0.81 mg · L–1 as Aℓ)
- reduces ( M-alk. ) by 0.45°F and increase sulphates by the same amount;
- forms 2.35 mg · L–1 of Aℓ hydroxide;
- releases 4 mg · L–1 of CO2
and, in the event of an excessively low ( M-alk. ), in the above example, we have to add 3.33 mg · L–1 (0.45 °F) of hydrated lime Ca(OH)2 in order to keep the pH within the optimum zone (see also section Neutralisation – remineralisation).
When using aluminate, on the other hand, we may have to use an acid to neutralise its sodium alkalinity; additionally, the reagent is expensive and this restricts its use to combined clarification/silicon removal (see precipating silica).
aluminium polymers
The preceding aluminium salts, if partially rendered alkaline by adding OH- before they are used, will "prepolymerise" hydroxide chains, leaving many Aℓ3+ sites available for coagulation.
Having the overall formula Aℓn(OH)p(Cℓ)3n–p (aluminium polychlorides) or Aℓn(OH)p(Cℓ)q(SO4)r (aluminium polychlorosulphates), these aluminium polymers can be identified by their molar ratio

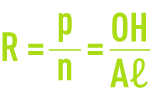
The higher this ratio, the longer the chains and the more effective the reagent’s flocculating capability, rapidly producing a dense (cohesive) floc of a good size; however, the greater R becomes, the more the product becomes unstable; in practice, R is usually restricted to the 0.4 to 0.6 range.
There are many commercially available products that are constantly being upgraded.
BAPC (basic aluminium polychloride)
If we want to increase R even further, the product has to be prepared immediately before use and then R can reach figures > 2, making rapid flocculation possible with a minimum treatment level and satisfactory removal of organic matter.
Unfortunately, the product is not easily prepared in situ and, up until now, this drawback has prevented the widespread use of this reagent.
iron salts
Ferric salts are the most frequently used iron salts. The overall reaction is the same as that for aluminium salts:

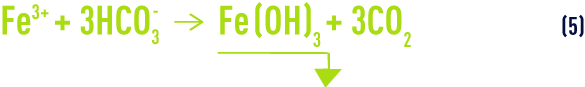
and, for instance, with ferric chloride in water with calcium alkalinity:


With iron salts, we have the same problems relating to the consumption of M-alk. and/or alkaline products that have to be added in order to maintain pH within the optimum range (which is, however, much more extensive than the range applicable to Aℓ salts).
The following forms are commercially available:
- sublimated FeCℓ3 or crystallised FeCℓ3 iron chlorides, 6H2O and the most frequently liquid at 600 g · L–1 of FeCℓ3 (41 % by weight);
- crystallised iron sulphate Fe2(SO4)3 in powder form, 9H2O and even chlorinated copperas;
- ferrous sulphate FeSO4 in powder form, 7H2O; however, this reagent has to be oxidised (Fe2+ Fe3+), often by Cℓ2, before use at a pH close to neutral; furthermore, FeSO4, an industrial by-product, is rarely of the grade required for drinking water or even industrial water processing.
Similarly, reaction (6) allows us to anticipate the fact that 10 mg · L–1 of a 41% commercial solution of ferric chloride, for instance, will lower M-alk. by 0.38°F, increase chlorides by the same amount, form 2.7 mg· L–1 of Fe(OH)3 and release 3.3 mg · L–1 of CO2.
The absence of iron prepolymer on the market should be noted.
On the other hand, the ferrate ion (sodium ferrate or preferably potassium ferrate because the latter is more stable) does have potential value because it has a dual action:
- oxidising, the hexavalent iron in the FeO42– ion will accept electrons so that can be reduced to Fe(III);
- coagulating, using the Fe3+ ion that is obtained.
When dissolved in water, this product will create oxygen and OH– ions (leading to the need to adjust the pH and, if necessary, to use it alongside a classic coagulant that has an acidifying effect).
customary treatment rates
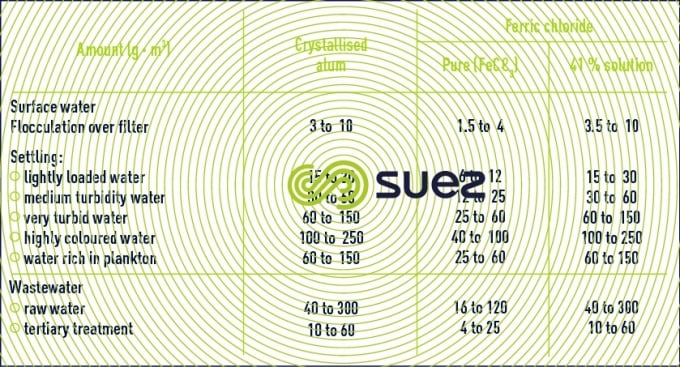
Tests are usually required before selecting the best reagent (quality/price) and to determine the level of treatment required.
Table 6 provides a rough guide to the amounts of iron or aluminium salts, expressed in g · m–3 (or mg · L–1), to be used in clarification treatment.


"natural" flocculation additives
mineral flocculants
activated silica
Activated silica was the first flocculent to be used. It produces good results mainly when combined with aluminium sulphate in cold water. Not very stable, activated silica must be prepared immediately before use by partly neutralising the alkalinity of a sodium silicate solution.
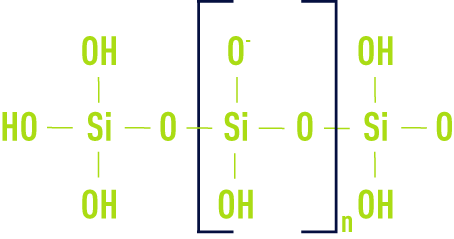
H2SO4 is the customary acidifying agent but we can also use HCℓ, NaHCO3, chlorine water … Subject to precise pH and concentration conditions, silicic acid released Si(OH)4 will polymerise by forming Si – O – Si bonds, first producing a dimer (OH)3 Si – O – Si(OH)3 and then an ionised polymer having the following general formula:

0.5 to 4 mg · L–1 expressed in SiO2. is the amount required for treatment purposes.
aluminosilicate
Acid, alum in solution can be used to activate sodium silicate. This method produces a polymerised aluminosilicate. There are also composite products available through the trade and used for coagulation and flocculation. These products are manufactured by injecting polysilicic acid into an aluminium salt to form, depending on the case, aluminium polysilicosulphate (PASS) or aluminium polysilicochloride (PASIC).
other mineral additives
Upstream from a sedimentation or filtration stage, some products are used to load raw water that is deficient in suspended solids. These products are not flocculants but contribute to floc growth and densification.
These will include, for instance:
- some clays (bentonite, kaolin);
- powdered calcium carbonate;
- powdered activated carbon (mainly used as an adsorbent);
- fine sand (so-called "ballasted floc" techniques, see section ballasted floc sedimentation).
organic flocculants (natural polymers)
These are natural polymers extracted from plant or animal substances.
alginates
Sodium alginates are obtained from alginic acid which is itself extracted from marine algae. The main components of this polymer structure are mannuronic acid and guluronic acid. Their gramme-molecular weight ranges from 2·103 to 104 D.
The amount to be used in treatment will range from 0.5 to 2 mg · L–1.
starches
Starches are obtained from corn, potato, tapioca or extracts from plant seeds. Starches are branched and not linear glucopyranose polymers that are sometimes degraded (OH–) or derivatives (carboxy-ethyl-dextrone). They are applied at the rate of between 1 and 10 mg · L–1, preferably in conjunction with aluminium salts.
Starches and alginates are solid products that have to be prepared as a solution at concentrations of 5 to10 g · L–1. Once they have been diluted, they can biodegrade rapidly, especially at temperatures above 20°C.
other compounds
Several natural polysaccharides (derived from cellulose, gums, tannins).
synthetic organic coagulants
These are synthetic organic molecules that are cationic in nature, having a medium gramme-molecular weight (104 to 105 D). They are only available as a liquid, in a water based medium.
These products can be used as supplied (no need for a preparation unit), wholly or partly replacing a mineral coagulant. They must be injected after in-line dilution.
An organic coagulant will only have a marginal effect on pH and injects very little additional salinity. The use of organic coagulants will significantly reduce the sludge production. Sludge extracted will be denser but also more ‘sticky’. Therefore, they cannot be adapted for use in all separation structures.
classification
There are three main groups:
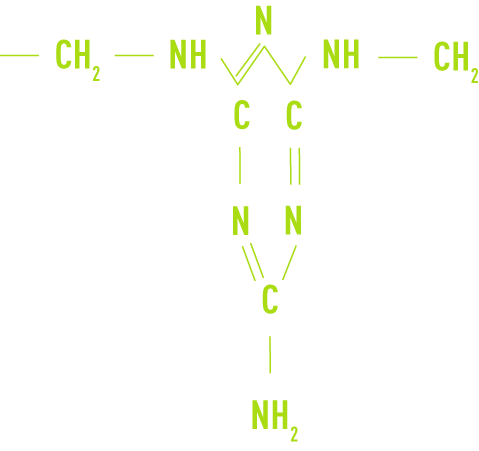
- melamineformaldehyde (or melamineformol)

- epichlorhydrine dimethylamine (or epi.DMA)

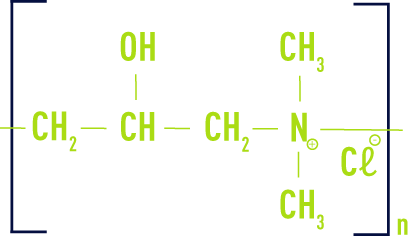
- poly(chloride diallyldimethyl-ammonium) (or polyDADMAC)

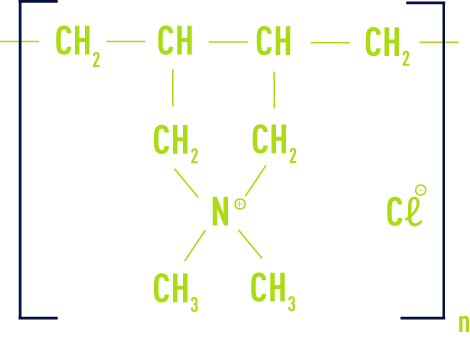
There are also other products such as polyamines that differ from epi.DMA and the polyethylenimines, and that are mainly used in industrial wastewater clarification.
Note: There are also organic coagulants obtained from natural sources (e.g. Chitosan, extracts of seeds from tropical trees…), but these are rarely used.
areas of application
Given the limited number of cationic charges that can be used, organic coagulants are mainly of interest when dealing with fine suspended solids or lightly laden colloids (low zeta potential) where their use is simple and cost-effective.
clarification
When treating water intended for food purposes, verify the legislation for the country concerned. The amounts of treatment chemicals to be applied range from 2 to 15 g · m–3 expressed as the commercially available liquid product.
coagulation over filter
In seawater filtration applications, the coagulation reaction can be combined with a premature and prejudical reaction involving precipitation of the coagulant due to the high salinity level. Therefore, only a few coagulants will be suitable.
industrial waste water
This is an important area of utilisation for organic coagulants. The treatment level (5 to 50 g · m–3) will depend to a large extent on the type of effluent required.
synergy with a mineral coagulant
In some cases, organic coagulants used on their own will not enable us to achieve the quality of water produced by a mineral coagulant.
The combined use of these two coagulants means that we can significantly reduce the amount of mineral coagulant required (40 to 80%), while producing smaller quantities of sludge.
synthetic organic flocculants
These are very long chain macromolecules obtained by polymerising synthetic monomers, some of which have electric charges or ionisable groups. These are very high gramme-molecular weignt products (106 to 107 D) that make remarkable performances possible (size, solidity, floc density), usually considerably better than those generated by natural polymers.
classification
Each polymer is classified on the basis of its ionicity.
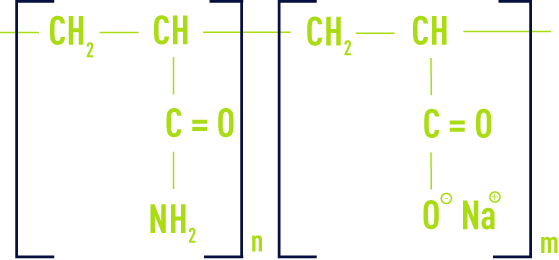
anionic
These products are normally acrylamide and acrylic acid copolymers.

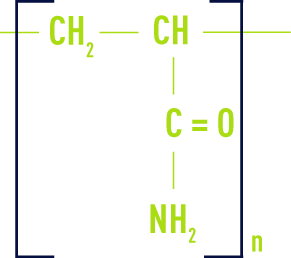
neutral (or non-ionic)
These are primarily polyacrylamids.

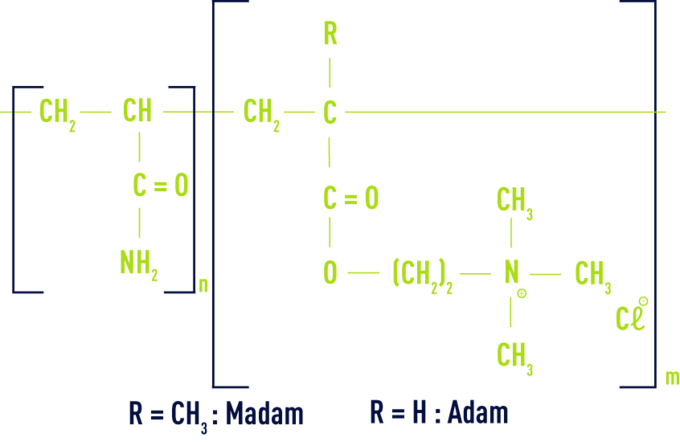
cationic
These are acrylamide and chloromethylated and quaternised N cationic monomer copolymers, usually MADAM (dimethylamino-ethyl methacrylate) or ADAM (dimethylamino-ethyl acrylate).

utilisation
Organic flocculants come in three forms:
- solid;
- emulsion (polymer in an organic solvent emulsion);
- solution (approximately 20 g · L–1 in a water-based medium).
The use of solid or emulsion flocculants always requires a specially prepared solution. These products always require secondary dilution (see section using “polyelectrolyte” polymers).
areas of application
surface water
In clarification applications, the synthetic flocculant will be used in conjunction with a coagulant. The best polymer will usually be anionic and, more rarely, a non-ionic or very slightly cationic polymer.
Typical amount ranges from 0.05 to 0.5 g · m–3. In some particularly highly loaded water (desludging), up to 2 g · m–3 of polymer may be required.
(Attention: in most countries, the use of polymers in drinking water treatment is regulated, see section pollution generated by water treatment).
industrial wastewater
When using a mineral coagulant treatment, up to 2 g . m–3 of an anionic polymer will generally be required. In some special cases (surface treatment, steel mills, gas washing), a cationic coagulant-flocculant polymer on its own is often the best solution (0.5 to 5 g . m–3).
urban wastewater (physical-chemical treatments)
When used in conjunction with a mineral coagulant, the anionic flocculant tends to be the best option. When the only requirement is the removal of suspended solids, a synthetic flocculant can be used on its own.
sludge dewatering
Cationic flocculants are usually suitable for organic sludge processing. Mineral type sludge requires anionic flocculants. Between 0.5 and 10 kg of polymer will be required per tonne of dry solids (see chapter Liquid sludge treatment).
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later














