other precipitations (case of anions)
Reading time:sulfates
Sulphate precipitation may be compulsory before discharging into the drains (affecting concrete) or if the water is to be re-used (scaling) and even to comply with certain discharge requirements.
The process the most frequently used, and which involves high levels of SO42-, consists in the cold precipitation of gypsum CaSO4, 2H2O by adding Ca2+ as lime (frequent case for acidic water) or CaCℓ2(brackish water) according to the following reaction:

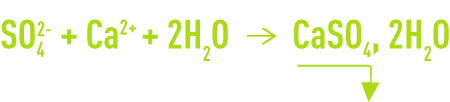
In both cases, precipitation as heterogeneous crystals is very slow. In order to avoid supersaturation and dangerous post precipitation, this reaction has to take place in the presence of a high concentration of seeds (> 20 g · L–1).
The SO42– concentrations obtained will depend on salinity, the medium’s ionic activity and the excess Ca2+; see section characteristics solution constants, figures 13 and 14; the diagrams in those figures provide approximate values for the solubility products obtained depending on the nature of the salts present.For instance, the following residual levels may be obtained:
- 2 to 3 g · L–1 SO42– in brackish water treatment applications, using CaCℓ2,
- 1.5 to 2.5 g · L–1 SO42– when neutralising acid water using lime without using CaCℓ2.
A second process is the precipitation of barium sulphate by adding BaCℓ2. The residual solubilities obtained are below 20 mg · L–1 but the reagent is extremely expensive and rarely used.
Permutation with a strong anionic resin SO42–/2Cℓ–can also be considered in intermediate cases at the cost of discharges (eluates) that are high in Cℓ– and SO42–
fluorides
The elimination of fluorides through precipitation involves the acid effluents produced by incineration gas scrubbing, aluminium (metallurgy) and also the phosphoric acid, glass and electronics industries. In the last three cases, the presence of F– will always be linked to a high level of silica which will always alter precipitation conditions. Lime will always be used as the neutralising agent, possibly supplemented by CaCℓ2, if we need to obtain low residual levels of F–:

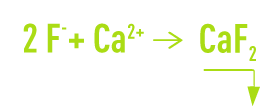
With a pollution that is primarily fluorinated and sulphuric or hydrochloric, the precipitate will be based on CaF2 whose crystallisation and precipitation will have kinetics that lie between those of CaCO3 and gypsum. These units have dimensions that are close to those of units used for carbonate removal but reaction times will be extended.

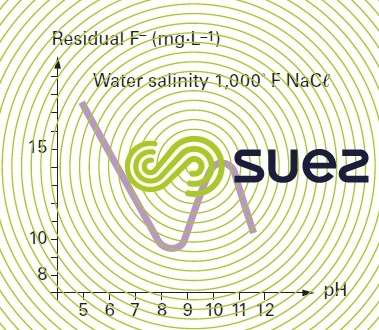

In precipitation mode, adding only lime, CaF2 solubility will depend on pH for a given salinity (figure 8). It depends on other factors and, therefore, also on the process originating these effluents. Thus we obtain:
- from 16 to 30 mg · L–1 in galvanoplasty effluents;
- from 2 to 5 mg · L–1 in effluent produced from the manufacturing of phosphoric acid, but using F– adsorption over precipitated hydroxyapatite;
- from 20 to 40 mg · L–1 in saline effluents. Dissolved aluminium in noticeable concentrations is an adverse sequestering element. Conversely, in the event of a massive coprecipitation of Aℓ(OH)3 or Mg(OH)2, these hydroxides adsorb fluorides and can decrease residual solubility.
Based on an Aℓ/F = 1 ratio, this adsorption will produce fairly low F– residual levels (2 to 4 mg · L–1).
When there is a major silicon presence, a parallel reaction takes place:

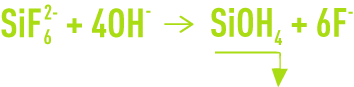
The F– concentrations obtained depends on the Ca2+ enrichment of the medium.
Copious and highly hydrated precipitation of silica gel takes place slowly and sludge thickening will be the factor that determines the settling tank capacity.
phosphates
These salts can be found in water in a variety of forms and concentration levels:
- phosphoric acid in phosphated fertiliser factory effluent with HF and SiO2;
- phosphates in household wastewater;
- phosphates in boiler blowdown;
- polyphosphates and hexametaphosphate in cooling circuits (see precipitation inhibitors).
There are two potential precipitation modes:
- acid effluents: through lime;
- non-acid water: through Aℓ or Fe salts (formation of metal phosphates).
precipitation using lime
Depending on the initial acidity, there can be two reactions:
- precipitation of calcium dihydrogen-phosphate at an optimum pH of 6 to 7:


This compound settles quite rapidly but has a high residual solubility (130 to 300 mg · L–1 as P2O5 depending on temperature);
- precipitation of tricalcium phosphate at an optimum pH of 9 to 12:

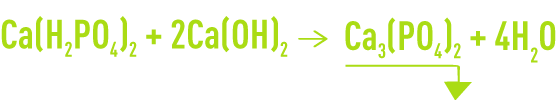
Tricalcium phosphate has a residual solubility of a few mg · L–1 as P2O5 but as a colloid. It settles slowly, even when a flocculent is added.
Magnesium, when it is present, has a more complex effect:
- below pH 9, calcium phosphate solubility increases with its concentration;
- above pH 10, calcium phosphate co-precipitates with magnesia with residual levels below 1 mg · L–1.
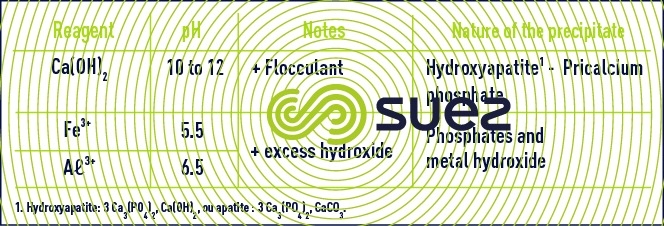
precipitation through Aℓ3+ or Fe3+
AℓPO4 and FePO4 are low-solubility salts but that will precipitate in their colloidal state. The precipitate is eliminated through flocculation over a metal hydroxide excess (figure 9).

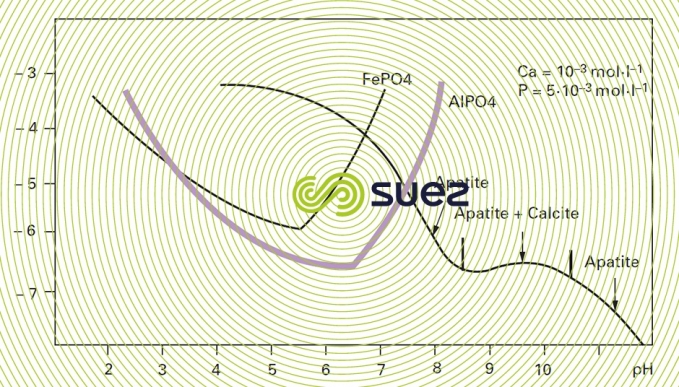

The residual values obtained for P can be significantly lower than one mg · L–1 by using relatively high amounts of iron or aluminium salts.


Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












