main types of ion exchangers
Reading time:ion exchanger characteristic
In order to be industrially applicable, an ion exchanger needs to meet a range of requirements:
- it must discharge the least possible polymerisation residue, measured through the TOC, especially in the case of food applications (resins approved by the health authorities), nuclear power stations and in ultra pure water applications;
- bead dimension and granulometry range must be calculated in such a way as to limit load losses from the exchanger columns.
The ion exchangers featured in the techniques described below use 0.3 to 1.2 mm diameter beads. Backflow applications, especially those involving blocked beds, often use single calibration resins (e.g. 0.65 mm).
For some special uses, resins will be crushed down to between 5 and 30 μm and delivered regenerated (condensate treatment). These micro-resins cannot be regenerated:
- the beads are mechanically and osmotically stable (minimum of broken or cracked beads).
During the exchanges, the exchanger may be required to fix ions or ionised complexes of varying sizes and weights.
In some cases, this will induce a contraction or an expansion that cannot be ignored (up to 100% for some carboxylic resins (HCO2-R) between phase H and phase NH4). This expansion and contraction, quite clearly, must not cause any of the beads to burst. Furthermore, in the most difficult cases, equipment dimensioning must allow for the need to permit this expansion to take place without generating excessively high compression strain within the layer.
The use of ion exchangers will be subject to a certain number of conditions:
- ion exchangers have been designed to fix ions and not to filter out suspended solids, colloids or oily emulsions. When these latter substances are present, they can only shorten the life of an ion exchanger;
- removal of soluble organic matter is a complex task and requires a special study;
- the presence of large quantities of dissolved gas in water can cause major disturbance to exchanger activity;
- powerful oxidants Cℓ2, O3, CℓO4 (perchlorate) damage resins;
- finally, the industrial exploitation of laboratory results or documents provided by ion exchanger manufacturers calls for proven experience.
Equipment design and operating rules are just as important as a knowledge of the theoretical performance of resins.
cation exchangers
Cation exchangers can be divided into two groups :
- highly acid cation exchangers;
- slightly acid cation exchangers.
highly acid exchangers
One of the characteristics of these exchangers is the presence of sulphonic radicals HSO3– that have an acidity similar to that of sulphuric acid. At present, these are sulphonated polystyrenes obtained from :
- styrene and divinylbenzene copolymerisation carried out in the form of an emulsion in order to obtain perfect spheres on solidification;
- sulphonation of the beads thus obtained.
The products created by this preparation are virtually single function products. Their physical and chemical properties vary according to the percentage of divinylbenzene to styrene, called the reticulation or crossing rate and which will usually vary from 6 to 16%.
For conventional fixed bed applications, the cationic exchange reticulation rate is approximately 8%.
In the case of high velocity (continuous or intermittent), short cycle treatments or the treatment of water containing traces of oxidants, resins with higher reticulation levels are used for both gel and macroporous structures.
low acid excahngers
These exchangers are polyacrylic resins characterised by the presence of carboxylic radicals HCO2, that can be likened to certain organic acids such as formic acid or acetic acid. They differ from high acid exchangers on two counts :
- they only fix cations Ca2+, Mg2+, Na+,Fe2+ ; Mn2+ … bonded to bicarbonates but cannot exchange cations that are in equilibrium with strong anions (SO42-, Cℓ–, NO3–);
- they regenerate easily (reaction of a strong acid on weak acid salts); the reaction is total, with regeneration levels that are close to the stoichiometry.
anion exchangers
We have:
- low alkalinity anionic exchangers;
- high alkalinity ion exchangers.
Their behaviour in the presence of acids will be different:
- low alkalinity ion exchangers will not fix very weak acids such as carbonic, boric acid, or silica unlike the high alkalinity exchangers that fix them totally;
- high alkalinity exchangers alone are capable of reacting with strong base salts (salt cut-off) according to reaction typee :


- low alkalinity exchangers are more or less sensitive to hydrolysis characterised by pure water displacing anions that had previously fixed on the resin:


whereas high alkalinity exchangers are virtually insensitive to this phenomenon;
- low alkalinity exchangers regenerate easily (reaction of a strong base with a weak base salt), the reaction is total with regeneration levels close to the Stoichiometry.
low alkalinity anion exchangers
These products are usually tertiary amines. Primary amines are rarely used and have very low alkalinity.
Their backbone usually has a polystyrenic or macroporous structure, or a polyacrylic structure Polyacrylic resins have a greater capacity and retain carbonic acid but are difficult to rinse.
high alkalinity anion exchangers
These products are quaternary amines. They usually have a polystyrenic or acrylic backbone with a gel or macroporous structure.
In the case of the polystyrenic variants, we have the higher alkalinity type 1 with trimethylammonium groups and type 2 with dimethylethanolammonium groups that usually have a slightly lower alkalinity level.
- Type 1: high alkalinity, strong affinity for silica and carbon dioxide, low capacity and poor regeneration performance.
- Type 2: lower alkalinity, lower affinity for silica and carbon dioxide, less chemically stable, higher capacity, better regeneration performance.
Polyacrylics have an alkalinity that is halfway between types 1 and 2. They are good at eluting organic matter but do not withstand temperatures of over 35°C too well.
There are also dual function polyacrylic resins that have both high and low functions in the same bead. These resins have a high exchange capacity but can only be used on water that does not contain much silica. Colloidal silica is not trapped by ion exchange resins.
a few figures
total capacity
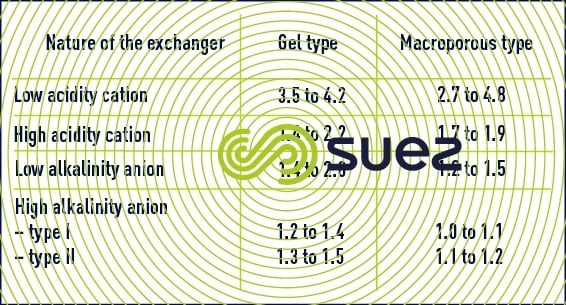
Table 21 provides an indication of the total exchange capacities for the various categories of exchangers expressed in equivalent-grams per litre of resin.


regeneration levels
These are only marginally dependant on the nature of the resin, but mainly on utilisation conditions; this explains the discrepancies recorded. The figures shown in table 22 are given in grams of pure product per litre of resin.



adsorbant resins and special resins
adsorbant resins
Using a system other than ion exchange and that is reversible, these products are designed to fix non-ionic compounds (mainly organic molecules) in solution in polar and non-polar solvents.
The phenomena involved in adsorption on solids are extremely complex and, therefore, the adsorption capacity of these resins depends on a number of factors of which the main ones are given below :
- the chemical composition of the backbone (polystyrenic, polyacrylic, formophenolic);
- the nature of the polar adsorbant functional groups (secondary amines, tertiary amines, quaternary ammonium);
- the degree of polarity;
- porosity (products that are usually macroporous having pores of up to 130 nm)
- specific surface area: up to 750 m2·g–1;
- hydrophylia;
- grain shape.
Potential applications are listed below:
- protecting ion exchange chains by fixing the pollution present in incoming water (humic acid, detergents…);
- removing colour from sugar syrups, glycerine, grape must, whey…;
- separation, purification, concentration in the pharmaceutical industry and synthetic chemistry.
The adsorbant resin regeneration mode mainly depends on the product adsorbed. Classic eluants are listed below: acids, bases, sodium chloride, methanol, appropriate organic solvents and, in some cases, pure water or vapor.
Choosing an adsorbant is not a simple task. Laboratories or pilot studies can often be essential.
special resins
chelating resins
These resins include special functional groups (aminophosphonics, aminodiacetic, aminodioxime, thiol) used to selectively fix heavy metals in different effluents (zinc, lead, mercury….), chromatographic metal separation, final softening of brackish water produced by electrolysis.
resins for nuclear usage
These products are more pure than resins employed in routine applications: 99% regenerated H+ form strong cationic resins, OH– strong anionic resins with less than 0.1% Cℓ–.
catalysing resins
- classic resins for base or acid catalysis (e.g. glucose inversion for the production of liquid sugar);
- resins with a metal catalyst (e.g. palladium resin used in demineralised or seawater de-oxygenation).
scavenger resins
These are strong basic anion-exchange resins which have a strong capacity to fix organic matter (10 to 15g of OM expressed in KMnO4) and which can be regenerated using alkalnine brine (NaOH + NaCℓ). These resins are mainly used in pretreatment processes in industrial water mineral removal units. Indeed, resins used at the beginning of a production cycle are in the form of Cℓ–.Consequently all anions present in the water to be treated, including hydrogen carbonates, are exchanged with Chloride ions. The use of this resin is therefore incompatible with drinking water production requirements or that of industrial water for use in the agri-food sector.
specific resins
For example : resins to treat nitrates (NO3). Nitrate treatment may be performed using a strong basic anion exchange ( SBA ) resin. Standard SBA resins have a greater affinity for sulphates than for nitrates. Resin manufacturers have therefore developed an SBA resin for this application which has a higher affinity for nitrates. Consequently, nitrates will be the last to leak. This resin is regenerated using NaCℓ and as for scavenger resins, ths resin is in the form of Cℓ- at the beginning of the production cycle. Consequently all anions present in the water to be treated, including hydrogen carbonates, are exchanged with Chloride ions. Therefore it is necessary to check that nitrate treatment does not cause permissible chloride content levels to be exceeded. Furthermore, the total fixation of alkalinity at the beginning of the cycle and then its partial fixation, leads to a lowering in pH as well as a modification in pH leves.
For example : Perchlorate (CℓO4–) treatment using resins. Perchlorate is a strong oxidant which is trapped by strong basic anion exchange resins ( SBA ) but which irreversibly destroys the resin. The resin cannot therefore be regenerated. Like the nitrate resin, the (CℓO4–) 4 resin at the beginning of the production cycles is in the form of Cℓ-. Consequently all the anions present in the water to be treated, including hydrogen carbonates, are exchanged with Chloride ions. This cycle, where anions permutate into Chloride, is a function of the salility of the water to be treated. It is relatively short (1 day) compared to the cycle for removing (CℓO4–) 4 (6 to 12 months) for contents of < 15 ppb and for permissible leakage of 4 ppb. Given the very long production cycle, OM content is a limiting factor.
Other examples: resins to treat boron. This strong basic anion exchange resin ( SBA ) is selective to boron. It enables water containing a few mg/L of boron to be treated. Regeneration is affected by acid (HCℓ or H2SO4), soda or ammonia. This resin can be used for desalination post-treatment or for the production of ultra-pure water in industry. For the record, in 2015 the WHO standard on boron content in drinking water stood at < 0.05 mg/L, the EC standard is less restrictive at <0.1 mg/L.
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












