catalytic ozonation : toccata
Reading time:The purpose of this process is to totally or partially degrade the soluble organic COD that is resistant to biological treatment. This application concerns:
- either residual pollution at a biological treatment outlet;
- or more or less concentrated effluent containing toxic pollutants (antibiotics, pesticides…) that make any biological treatment impossible.
The Toccata process, an heterogeneous catalytic process, consists of creating in a reactive medium a secondary oxidant from ozone that is more powerful than ozone alone (unstable species formed from the reaction between ozone and a solid catalyst). This reaction takes place under temperature and pressure conditions close to ambient. Therefore, it is one of the processes called advanced oxidation (see the oxidants and disinfectants). The oxidation of organic compounds is the result of three phases coming into contact: water to be treated, solid catalyst and ozonated gas.
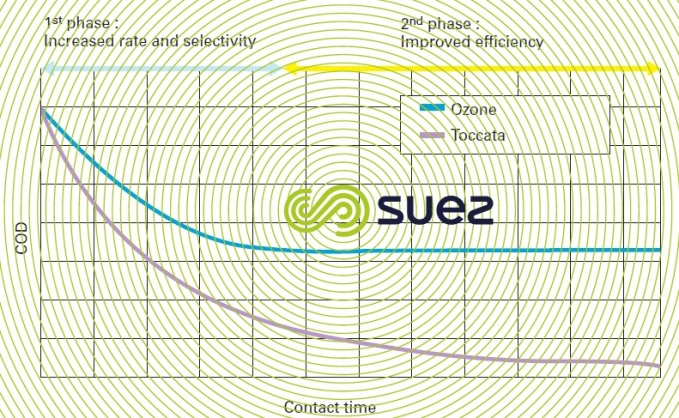
Catalytic ozonation ensures that COD is steadily eliminated without the appearance of any reaction limits like those normally encountered during ozonation when high levels of removal are targeted. Compared with ozonation alone, this process has two beneficial effects as illustrated in figure 28:
- increased COD removal rate and selectivity as expressed by the ratio between the amount of COD removed and the amount of ozone consumed during the first phase where ozone alone is active;
- improved oxidation performance during the second phase where ozone consumption can drop to zero in conventional ozonation.


From a chemical standpoint, catalytic and conventional ozonation processes when used to oxidise organic compounds will form other compounds that have a lower molecular weight with a polar and oxygenated chemical function such as aldehyde, ketone and carboxylic acid type.
Therefore an increased biodegradability results from the partial oxidation of the non-biodegradable COD when the rate at which biodegradable compounds disappear is lower than the rate at which non-biodegradable compounds are removed. In this case, it is suggested to use catalytic ozonation in conjunction with a subsequent biological treatment (Biofor, for instance).
The catalyst developed for this process is available in two forms that are appropriate for different reactors selected based on their application:
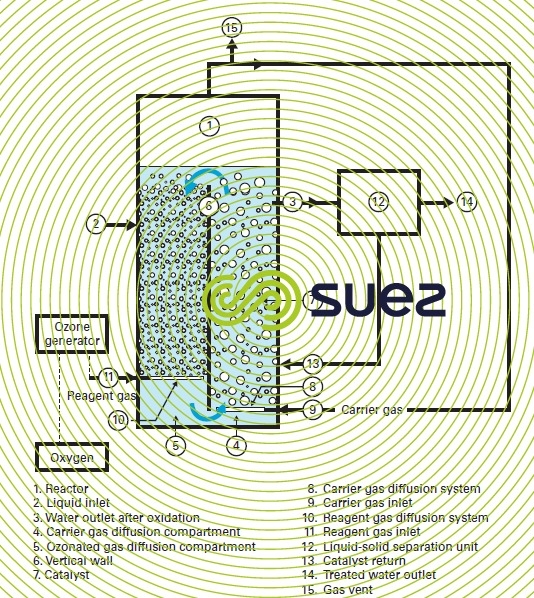
- A powder having an average particle size equal to a few hundred microns; before it can be used, it needs to form an homogenous suspension in a "moving bed" type reactor into which ozone is injected in the form of fine bubbles (figure 29 and photo 13). The reactor comprises two communicating compartments; the catalyst suspension circulates continuously between these compartments under the action of an incoming carrier gas (usually air) injected in a closed loop inside of the compartment. Ozonation takes place in the second compartment where the reagent gas containing ozone is injected against the flow. The ozone flux is controlled by the reaction rate in the effluent without taking into consideration any hydraulics as the catalyst needs to be maintained in suspension. The catalyst is continuously recycled into the reactor following solid-liquid separation (settling, micro-straining, hydrocyclone, membrane…):
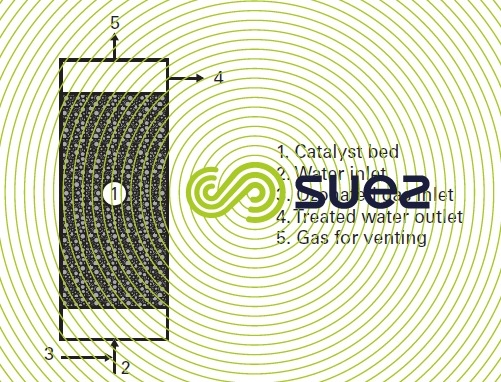
- Particles measuring a few millimetres in diameter suitable for use in a conventional fixed bed reactor fed with a rising co-current of water and ozonated gas (figure 30).







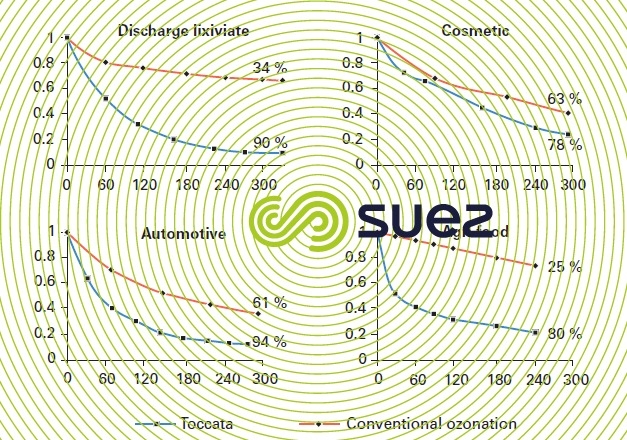
The Toccata process can be used to treat IWW from various sources with the goal to obtain high oxidation efficiencies (see examples given in figure 31). Unlike conventional ozonation or oxidation processes using radicals such as O3/H2O2 or O3/UV, the quality of the water to be treated has a relatively low effect due to the fact that process performance is controlled by the catalyst activity and is not affected by the inhibiting influence of compounds often present in IWW that capture the free radicals. Compared with conventional ozonation, the catalyst activity and selectivity will allow noticeable reductions in the amount of ozone transferred for the same level of COD reduction (refer to table 12 for examples).





Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












