applications
Reading time:Ozone has a very wide range of applications due to its powerful reactivity, (see the oxidants and disinfectants). In the water treatment industry, this range extends from drinking water treatment to the purification of industrial wastewater.
in drinking water treatment
Ozone’s action is used to advantage at various stages throughout production, depending on the quality of the water to be treated. surface water treatment systems feature several tables and examples of ozone applications.
in pre-oxidation
In pre-oxidation, ozone is used to break down the structure of colloidal particles and of macromolecules, thus enhancing coagulation-flocculation performances and even sedimentation operation. Clarified water quality will be better in terms of turbidity and organic matter (TOC, compounds that are haloform precursors, compounds responsible for taste and odour). At the same time, pre-oxidation will eliminate algae present in surface water. Pre-oxidation also oxidises iron and manganese found in groundwater that has a low organic matter content. At this stage, approximately 1 mg · L–1 is used.
in intermediate or main oxidation
With intermediate or main oxidation, the purpose of ozone is to oxidise naturally occurring organic matter. Ozone removes color from water containing humic substances. It transforms organic compounds encountered such as those responsible for taste and odor (e.g. geosmine and 2-methylisoborneol), pesticides (glyphosate, aldicarb, pentachlorophenol…), phenols, detergents, algal toxins and other chemical compounds of pharmaceutical origin and/or having an oestrogenic effect (e.g. 17a- ethinylestradiol). The extent of the conversion of the compounds outlined above varies according to their chemical structure and environmental conditions. The radical reactivity method, which is recommended by simply adding hydrogen peroxide can prove necessary in order to obtain the removal rates targeted.
This type of advanced oxidation is subject to authorisation because the by-products formed can fall within the scope of regulations (case of pesticides and bromates in Europe). As a rule, ozonation reduces the toxicity of compounds found in water and enhances their biodegradability (BDOC). Consequently, a main oxidation process via ozonation is almost always followed by a biological filtration on granular activated carbon in order to fine tune the removal of micro-pollutants while restricting the amount of ozone applied. This amount tends to range from 0.5 to 1 g per g. of organic carbon.
in disinfection
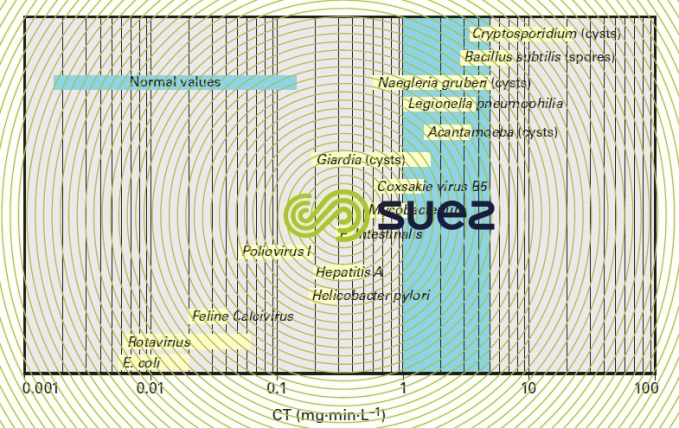
In disinfection, because ozone reacts rapidly with micro-organisms the CT values are low (see the oxidants and disinfectants). The range of CT values compiled in figure 22 show that ozone is capable of destroying the commonly known pathogenic organisms. Despite the fluctuation observed with these CT values due to varying experimental conditions and count methods (encystations, infectivity) the following observations can be made:
- the most resistant micro-organisms to ozone are the protozoa such as Naegleria and Cryptosporidium cysts involving CTs that are in excess of 100 times higher than those required for the E. coli bacterium;
- a 2 mg · min · L–1 CT will be enough to destroy 99% of bacteria, viruses and all Giardia cysts.


As in the case of any chemical reaction, the action of ozone on micro-organisms depends on the conditions prevailing in the medium such as pH, temperature, the presence of other oxidisable compounds and suspended solids concentration. In fact:
- the pH governs ozone breakdown; as it increases in the base range. A rising pH has the effect of reducing the dissolved ozone concentration and, accordingly, disinfection effectiveness;
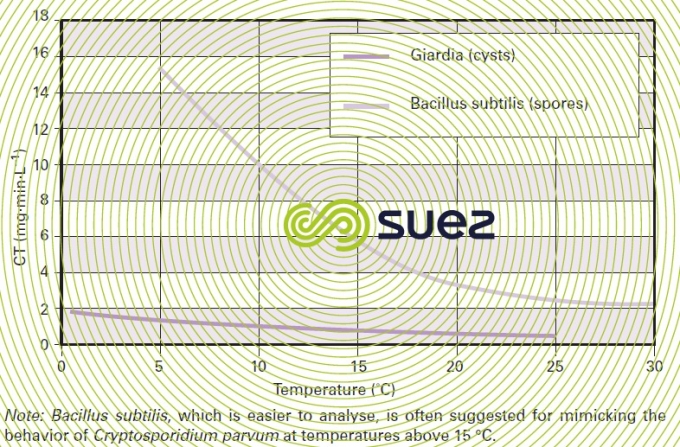
- although temperature has the opposite effect on ozone solubility and on the inactivation rate, disinfection effectiveness increases with temperature (figure 23);
- organic matter contributes to water ozone demand and competes with the micro-organisms (ozone demand: see specific analysis). Residual dissolved ozone only appears in the water when the amount of oxidant injected rises above a critical value corresponding to the instant ozone demand;
- suspended solids can protect micro-organisms and therefore render disinfection more difficult (this holds true regardless of the disinfection agent used).


The CT values to be maintained depend on the purpose of the disinfection. It is accepted that the drinking water treatment conditions defined in 1964, i.e. 0.4 mg · L–1 of residual ozone for a 4 minutes contact time (CT = 1.6 mg · min · L–1) will meet the target of 2.5 log Giardia lamblia cyst elimination at 5°C. The most advanced studies tend to demonstrate that a one log inactivation of Cryptosporidium cysts would require a minimum CT of 4 mg·min·L–1 at 20°C and 10 mg · min · L–1 at 10°C (compilation carried out by the USEPA). Within this context, the use of ozone may fail to satisfy the standards on bromate ions (see below).


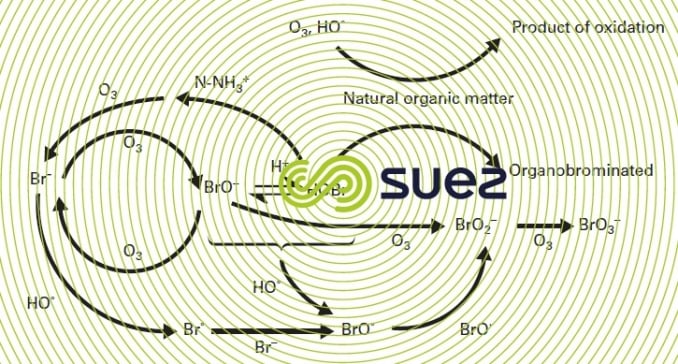
The formation of bromates during ozonation is caused by the oxidation of bromide ions according to a complex mechanism involving ozone and the hydroxyl radicals (figure 25). In natural waters where the pH ranges between 6 and 8, ozone plays a key role in the formation of hypobromous acid (HOBr) which is then oxidised by the hydroxyl radical (HO•) to form bromite ions (BrO2–) in turn easily converted into bromate ions (BrO3–) by ozone. The formation of organobromate compounds is a minor phenomenon. Consequently, converting bromide ions into bromate ions can be minimised by:
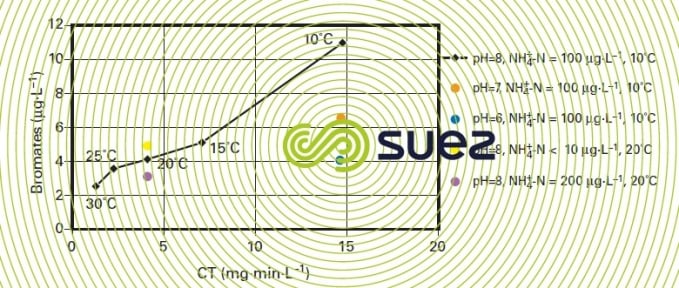
- controlling pH in order to limit ozone breakdown into a hydroxyl radical (see the oxidants and disinfectants) and influencing the HOBr/BrO– balance (figure 24, influence of the pH at CT = 15) ;
- adding ammonia that reacts very quickly with hypobromous acid;
- controlling the contactor hydrodynamics by adjusting ozone application conditions.
It is possible to anticipate changes in bromate concentration if one knows the initial bromide concentration, the dissolved ozone concentration, pH, temperature and contact time.


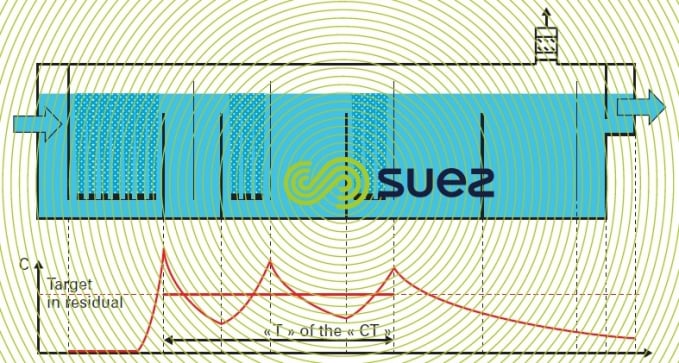
When producing drinking water, the main ozonation reactors are chambers made up of compartments. One single compartment with a contact time of a few minutes is usually enough to provide pre-oxidation whereas main oxidation and disinfection are achieved with two or three compartment chambers and contact times of 8 to 15 minutes. These reactors are sized based on the following considerations:
- the model of completely mixed reactors for each gas diffusion compartment and plug-flow reactors between the baffle is used to characterises the reactor actual distribution of residence times. Hydrodynamic effectiveness is evaluated in terms of T10 determined following tracer testing (see applications);
- kinetic parameters (instant ozone demand, subsequent ozone consumption rate in order to obtain a residual concentration) can be ascertained via laboratory testing or by analogy.
Mass balance calculations on ozone allow determination of the operating parameters: gas flow rate through each compartment based on geometric properties and operating limitations (ozone residual concentrations targeted for disinfection, oxidation treatment objective, see illustration provided in figure 26).


The U tube is also used for the main oxidation process (photo 11). The radial diffuser reactor shown in figure 18 is also particularly well suited to pre-ozonation.



municipal wastewater
The use of ozone in wastewater treatment applications meets various objectives.
After physical-chemical or biological treatment, ozonation is used to:
- disinfect treated water while reducing residual COD by at least 20% prior to re-use or to discharge in protected areas;
- remove color and eliminate 4 to 8 mg · L–1 of detergents from water with approximately 30 mg · L–1 ozone (combined textile IWW and UWW ).
The reactors are mainly compartmented ozonation chambers designed similarly to those used for drinking water ozonation. In disinfection, because bacteria or virus inactivation occurs very rapidly and no residual ozone concentration is required, a contact time of two minutes or less is enough to ensure the chamber complete disinfection. This application is also suitable for use with static mixer systems.
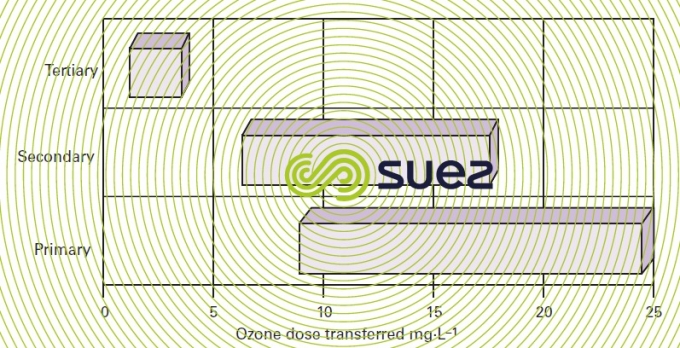
Disinfection effectiveness is often evaluated in terms of the amount of ozone transferred to the water to inactivate fecal coliforms or E. coli. This effectiveness improves as the quality of water improves with reduced levels of oxidisable compounds while it progresses through the plant treatment strain. Figure 27 illustrates the fluctuation of the ozone dose according to the different primary,secondary or tertiary effluent types. As a rule, treating a primary effluent is unreasonable; the least required is a good quality secondary effluent (BOD < 20 mg · L–1 and suspended solids < 20 mg · L–1) or otherwise a minor reduction in bacteria or viruses (less than 2 log) should be expected.


In activated sludge treatment processes, injecting ozone through an external loop reduces sludge production (see Biolysis O) while improving the quality of the biological floc (see reduced sludge production). The reaction is fast and requires a completely mixed reactor with a strong and non-conventional agitation, that creates an extensive gas liquid exchange area.
When treating excess biological sludge delivered by anaerobic digestion or by aerobic stabilisation, ozone will improve sludge dewatering and thickening, thereby having a beneficial effect on odor emission.
odor control and gas scrubbing
(see measuring odours and odour control)
Ozone is used to oxidise sulphur compounds (sulphides and mercaptans such as hydrogen sulphide and methyl mercaptans), amines (e.g. ethylamine and trimethylamine), ketones, alcohols and aldehydes. A few cases of industrial application exist. These are based on the use of scrubbing towers consisting of packing bed columns in which wet oxidation takes place (figure 20). Contact time is approximately 3 seconds. Wash water pH is set at 9-9.5 for the removal of sulphur compounds, aldehydes, ketones and alcohols, and to below 6.5 to remove amines. The average amount of ozone applied varies between 6 and 12 mg · m–3 of TPN gas.
swimming pools
Ozone is used at various stages in the treatment of recycled water in watercenters :
- injected at the rate of 0.5 to 1 mg · L–1 in the buffer tank at the outlet of the basin, it oxidises anthropogenic contaminants (amine substances);
- subsequently applied between two filtration stages, it oxidises residual organic matter, thus improving the visual and organoleptic quality of the water and disinfecting it;
- injected upstream of the basin, it forms a bactericidal and virucidal barrier.
The reactors normally used are bubble columns. Static mixer systems are suitable for treatment upstream of filtration.
industrial wastewater
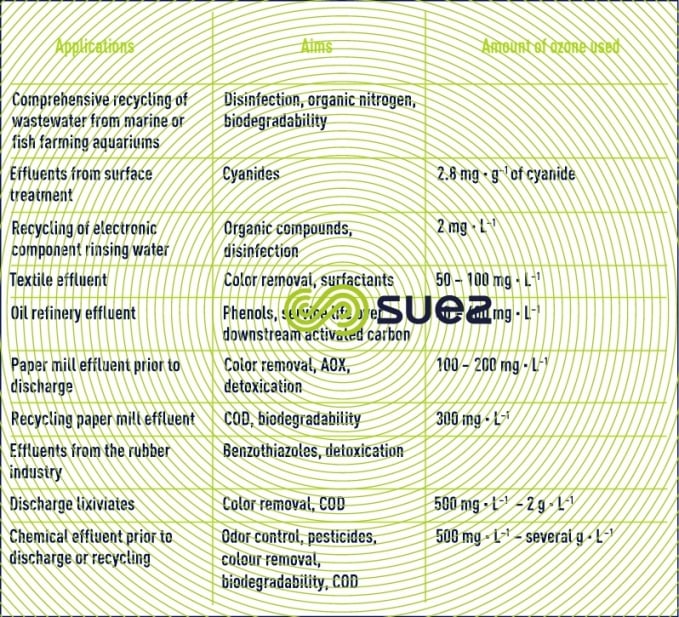
There are many applications for the use of ozone in industrial wastewater treatment as demonstrated by a recently prepared list of plants that are currently under operation and displayed in table 11. The amounts of ozone referenced are extremely variable depending on treatment objectives and quality of the water to be treated.


For each application, the reactor is selected according to reaction rate and water quality. For instance, it is a packing bed column on a fraction of the feed flow or recycling flow in fish farming for disinfection, a static mixer system to remove color from a relatively clean water or a radial diffuser reactor to treat effluent with a long contact time (photo 12). These units are sized following a chemical kinetic study in continuous or intermittent mode.



industrial applications
Ozone’s reactivity is put to good use in various stages of industrial production:
- to whiten paper pulp (see paper mills and the paper pulp industries);
- to purify (whitening) kaolin and calcium carbonate;
- to chemical syntheses, flavourings, organic acids …;
- to purify sulphuric and phosphoric acids;
- to whiten cane sugar;
- to condition food products.
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later













