domain of application
Reading time:Chlorine dioxide, an extremely oxidant, has high disinfectant, color removal and odor control capacities.
As a disinfectant, it acts much faster than chlorine on micro-organisms, especially on protozoa (table 4); it also has a better remanent effect.



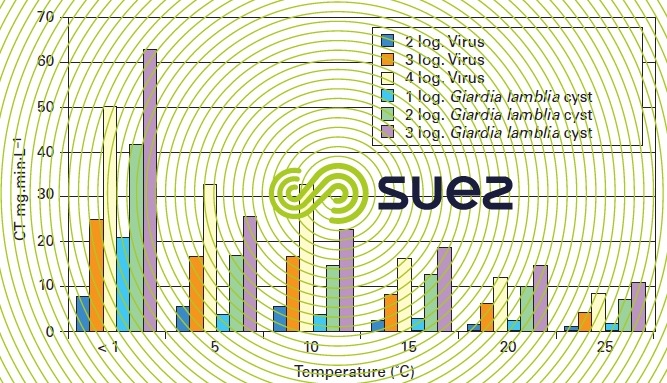
As in the case of chlorine, the effectiveness of chlorine dioxide used in disinfection depends on temperature as demonstrated by the accepted CT values for Giardia Lamblia and virus inactivation (figure 5).


It does not produce sapid compounds, nor does it create halogenic by-products. Therefore, it should be used instead of chlorine when the untreated water contains traces of phenols likely to combine with chlorine and produce unpleasant tasting water because of chlorophenol formation. On the other hand, chlorine dioxide contains chlorates which, when reduced to chlorites, gives an unpleasant metallic taste to water.
Chlorine dioxide rapidly oxidises iron salts that can then precipitate as non-soluble ferric hydroxide. Similarly, when used in excess amounts that vary with the water pH, it oxidises manganese salts to produce manganese dioxide.
Chlorine dioxide is used with drinking water in limit cases :
- occasionally for pre-oxidation when water contains little organic matter, otherwise chlorine dioxide reduction during organic compound oxidation will produce toxic chlorite ions;
- in disinfection, the presence of a 0.2 mg · L–1 residual concentration at the end of the plant treatment train is usually sufficient to safeguard the treated water microbiological quality in the distribution network. In the presence of organic matter the use of chlorine dioxide may be banned to prevent the formation of chlorite ions. Many countries have limited the amount of CℓO2 that can be used in disinfection, either directly by establishing a maximum amount of chlorine dioxide (0.8 mg · L–1 in the USA for instance), or indirectly by imposing a maximum chlorite and chlorate content in treated water (in Europe, this is equivalent to applying a maximum of 0.4 mg · L–1 of chlorine dioxide).
Bookmark tool
Click on the bookmark tool, highlight the last read paragraph to continue your reading later












